Page 22 - Read Online
P. 22
Page 10 of 14 Daniele et al. Hepatoma Res 2021;7:61 https://dx.doi.org/10.20517/2394-5079.2021.58
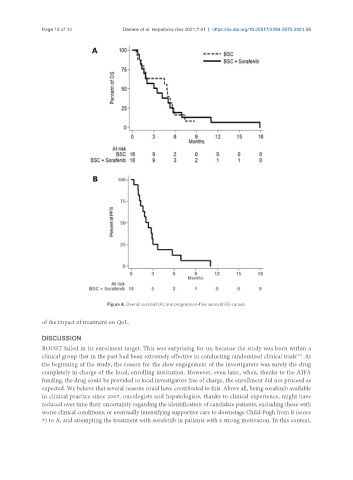
Figure 4. Overall survival (A) and progression-free survival (B) curves.
of the impact of treatment on QoL.
DISCUSSION
BOOST failed in its enrolment target. This was surprising for us, because the study was born within a
clinical group that in the past had been extremely effective in conducting randomized clinical trials . At
[16]
the beginning of the study, the reason for the slow engagement of the investigators was surely the drug
completely in charge of the local, enrolling institution. However, even later, when, thanks to the AIFA
funding, the drug could be provided to local investigators free of charge, the enrollment did not proceed as
expected. We believe that several reasons could have contributed to this. Above all, being sorafenib available
in clinical practice since 2007, oncologists and hepatologists, thanks to clinical experience, might have
reduced over time their uncertainty regarding the identification of candidate patients, excluding those with
worse clinical conditions, or eventually intensifying supportive care to downstage Child-Pugh from B (score
7) to A, and attempting the treatment with sorafenib in patients with a strong motivation. In this context,