Page 100 - Read Online
P. 100
Cadamuro et al. Hepatoma Res 2022;8:11 https://dx.doi.org/10.20517/2394-5079.2021.140 Page 5 of 16
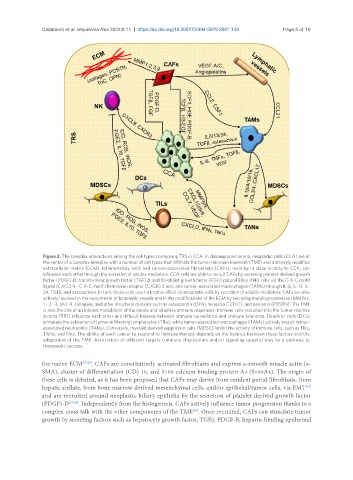
Figure 2. The complex interactions among the cell types composing TRS in CCA. In cholangiocarcinoma, neoplastic cells (CCA) are at
the center of a complex interplay with a number of cell types that infiltrate the tumor microenvironment (TME) and a strongly modified
extracellular matrix (ECM). Inflammatory cells and cancer-associated fibroblasts (CAFs), residing in close vicinity to CCA, can
influence each other through the secretion of soluble mediators. CCA cells are able to recruit CAFs by secreting platelet-derived growth
factor (PDGF)-D, transforming growth factor (TGF) β, and fibroblast growth factor (FGF); natural killer (NK) cells via the C-X-C motif
ligand (CXCL) 9 - C-X-C motif chemokine receptor (CXCR) 3 axis; and cancer-associated macrophages (TAMs) through IL-6, IL-13, IL-
34, TGFβ, and osteoactivin. In turn, these cells exert a trophic effect on neoplastic cells by secretion of soluble mediators. CAFs are also
actively involved in the recruitment of lymphatic vessels and in the modifications of the ECM by secreting metalloproteinases (MMPs)-
1, -2, -3, and -9, collagens, and other structural proteins such as osteopontin (OPN), tenascin C (TnC), and periostin (POSTN). The TME
is also the site of an intense modulation of the innate and adaptive immune responses. Immune cells recruited into the tumor reactive
stroma (TRS) influence each other in a difficult balance between immune surveillance and immune tolerance. Dendritic cells (DCs)
stimulate the activation of tumor-infiltrating lymphocytes (TILs), while tumor-associated macrophages (TAMs) actively recruit tumor-
associated neutrophils (TANs). Conversely, myeloid-derived suppressor cells (MDSC) inhibit the activity of immune cells, such as TILs,
TANs, and NKs. The ability of each cancer to respond to immune therapy depends on the balance between these factors and the
adaptation of the TME. Association of different targets (immune checkpoints and/or signaling targets) may be a pathway to
therapeutic success.
the native ECM [12,26] . CAFs are constitutively activated fibroblasts and express α-smooth muscle actin (α-
SMA), cluster of differentiation (CD) 10, and S100 calcium binding protein A4 (S100A4). The origin of
these cells is debated, as it has been proposed that CAFs may derive from resident portal fibroblasts, from
[15]
hepatic stellate, from bone marrow-derived mesenchymal cells, and/or epithelial/tumor cells, via EMT
and are recruited around neoplastic biliary epithelia by the secretion of platelet derived growth factor
(PDGF)-D [27,28] . Independently from the histogenesis, CAFs actively influence tumor progression thanks to a
complex cross talk with the other components of the TME . Once recruited, CAFs can stimulate tumor
[29]
growth by secreting factors such as hepatocyte growth factor, TGFβ, PDGF-B, heparin-binding epidermal