Page 74 - Read Online
P. 74
Lee et al. Hepatoma Res 2018;4:51 I http://dx.doi.org/10.20517/2394-5079.2018.78 Page 9 of 18
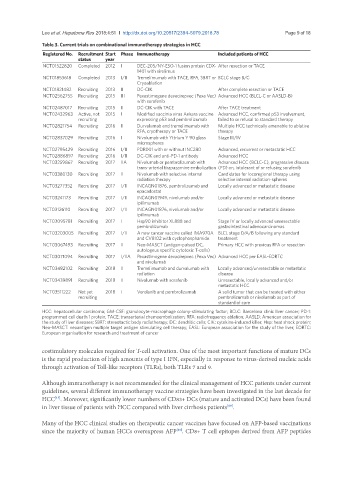
Table 3. Current trials on combinational immunotherapy strategies in HCC
Registered No. Recruitment Start Phase Immunotherapy Included patients of HCC
status year
NCT01522820 Completed 2012 I DEC-205/NY-ESO-1 fusion protein CDX- After resection or TACE
1401 with sirolimus
NCT01853618 Completed 2013 I/II Tremelimumab with TACE, RFA, SBRT or BCLC stage B/C
Cryoablation
NCT01821482 Recruiting 2013 II DC-CIK After complete resection or TACE
NCT02562755 Recruiting 2015 III Pexastimogene devacirepvec (Pexa Vec) Advanced HCC (BLCL-C or AASLD-B)
with sorafenib
NCT02487017 Recruiting 2015 II DC-CIK with TACE After TACE treatment
NCT02432963 Active, not 2015 I Modified vaccinia virus Ankara vaccine Advanced HCC, confirmed p53 involvement,
recruiting expressing p53 and pembrolizumab failed to or refusal to standard therapy
NCT02821754 Recruiting 2016 II Durvalumab and tremelimumab with Multiple HCC technically amenable to ablative
RFA, cryotherapy or TACE therapy
NCT02837029 Recruiting 2016 I Nivolumab with Yttrium Y 90 glass Stage III/IV
microspheres
NCT02795429 Recruiting 2016 I/II PDR001 with or without INC280 Advanced, recurrent or metastatic HCC
NCT02886897 Recruiting 2016 I/II DC-CIK and anti-PD-1 antibody Advanced HCC
NCT03259867 Recruiting 2017 IIA Nivolumab or pembrolizumab with Advanced HCC (BCLC-C), progressive disease
trans-arterial tirapazamine embolization (PD) on, intolerant of or refusing sorafenib
NCT03380130 Recruiting 2017 II Nivolumab with selective internal Candidates for locoregional therapy using
radiation therapy selective internal radiation-spheres
NCT03277352 Recruiting 2017 I/II INCAGN01876, pembrolizumab and Locally advanced or metastatic disease
epacadostat
NCT03241173 Recruiting 2017 I/II INCAGN01949, nivolumab and/or Locally advanced or metastatic disease
ipilimumab
NCT03126110 Recruiting 2017 I/II INCAGN01876, nivolumab and/or Locally advanced or metastatic disease
ipilimumab
NCT03095781 Recruiting 2017 I Hsp90 inhibitor XL888 and Stage IV or locally advanced unresectable
pembrolizumab gastrointestinal adenocarcinomas
NCT03203005 Recruiting 2017 I/II A new cancer vaccine called IMA970A BLCL stage 0/A/B following any standard
and CV8102 with cyclophosphamide treatment
NCT03067493 Recruiting 2017 II Neo-MASCT (antigen-pulsed DC, Primary HCC with previous RFA or resection
autologous specific cytotoxic T-cells)
NCT03071094 Recruiting 2017 I/IIA Pexastimogene devacirepvec (Pexa Vec) Advanced HCC per EASL-EORTC
and nivolumab
NCT03482102 Recruiting 2018 II Tremelimumab and durvalumab with Locally advanced/unresectable or metastatic
radiation disease
NCT03439891 Recruiting 2018 II Nivolumab with sorafenib Unresectable, locally advanced and/or
metastatic HCC
NCT03511222 Not yet 2018 I Vorolanib and pembrolizumab A solid tumor that can be treated with either
recruiting pembrolizumab or nivolumab as part of
standard of care
HCC: hepatocellular carcinoma; GM-CSF: granulocyte-macrophage colony-stimulating factor; BCLC: Barcelona clinic liver cancer; PD-1:
programmed cell death 1 protein; TACE: transarterial chemoembolization; RFA: radiofrequency ablation; AASLD: American association for
the study of liver diseases; SBRT: stereotactic body radiotherapy; DC: dendritic cells; CIK: cytokine-induced killer; Hsp: heat shock protein;
Neo-MASCT: neoantigen multiple target antigen stimulating cell therapy; EASL: European association for the study of the liver; EORTC:
European organisation for research and treatment of cancer
costimulatory molecules required for T-cell activation. One of the most important functions of mature DCs
is the rapid production of high amounts of type I IFN, especially in response to virus-derived nucleic acids
through activation of Toll-like receptors (TLRs), both TLRs 7 and 9.
Although immunotherapy is not recommended for the clinical management of HCC patients under current
guidelines, several different immunotherapy vaccine strategies have been investigated in the last decade for
HCC . Moreover, significantly lower numbers of CD83+ DCs (mature and activated DCs) have been found
[15]
[60]
in liver tissue of patients with HCC compared with liver cirrhosis patients .
Many of the HCC clinical studies on therapeutic cancer vaccines have focused on AFP-based vaccinations
since the majority of human HCCs overexpress AFP . CD8+ T cell epitopes derived from AFP peptides
[15]