Page 69 - Read Online
P. 69
Page 4 of 18 Lee et al. Hepatoma Res 2018;4:51 I http://dx.doi.org/10.20517/2394-5079.2018.78
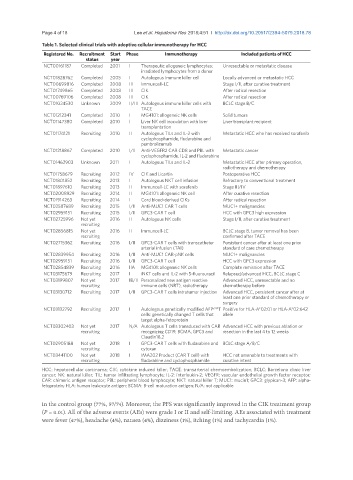
Table 1. Selected clinical trials with adoptive cellular immunotherapy for HCC
Registered No. Recruitment Start Phase Immunotherapy Included patients of HCC
status year
NCT00161187 Completed 2001 I Therapeutic allogeneic lymphocytes: Unresectable or metastatic disease
irradiated lymphocytes from a donor
NCT01828762 Completed 2005 I Autologous immune killer cell Locally advanced or metastatic HCC
NCT00699816 Completed 2008 III Immuncell-LC Stage I/II, after curative treatment
NCT01749865 Completed 2008 III CIK After radical resection
NCT00769106 Completed 2008 III CIK After radical resection
NCT01024530 Unknown 2009 II/III Autologous immune killer cells with BCLC stage B/C
TACE
NCT01212341 Completed 2010 I MG4101: allogeneic NK cells Solid tumors
NCT01147380 Completed 2010 I Liver NK cell inoculation with liver Liver transplant recipient
transplantation
NCT01174121 Recruiting 2010 II Autologous TILs and IL-2 with Metastatic HCC who has received sorafenib
cyclophosphamide, fludarabine and
pembrolizumab
NCT01218867 Completed 2010 I/II Anti-VEGFR2 CAR CD8 and PBL with Metastatic cancer
cyclophosphamide, IL-2 and fludarabine
NCT01462903 Unknown 2011 I Autologous TILs and IL-2 Metastatic HCC after primary operation,
radiotherapy and chemotherapy
NCT01758679 Recruiting 2012 IV CIK and Licartin Postoperative HCC
NCT01801852 Recruiting 2013 I Autologous NKT cell infusion Refractory to conventional treatment
NCT01897610 Recruiting 2013 II Immuncell-LC with sorafenib Stage III/IV
NCT02008929 Recruiting 2014 II MG4101: allogeneic NK cell After curative resection
NCT01914263 Recruiting 2014 I Cord blood-derived CIKs After radical resection
NCT02587689 Recruiting 2015 I/II Anti-MUC1 CAR T cells MUC1+ malignancies
NCT02959151 Recruiting 2015 I/II GPC3-CAR T cell HCC with GPC3 high expression
NCT02725996 Not yet 2016 II Autologous NK cells Stage I/II, after curative treatment
recruiting
NCT02856815 Not yet 2016 II Immuncell-LC BCLC stage B, tumor removal has been
recruiting confirmed after TACE
NCT02715362 Recruiting 2016 I/II GPC3-CAR T cells with transcatheter Persistent cancer after at least one prior
arterial infusion (TAI) standard of care chemotherapy
NCT02839954 Recruiting 2016 I/II Anti-MUC1 CAR-pNK cells MUC1+ malignancies
NCT02959151 Recruiting 2016 I/II GPC3-CAR T cell HCC with GPC3 expression
NCT02854839 Recruiting 2016 IIA MG4101: allogeneic NK cells Complete remission after TACE
NCT03175679 Recruiting 2017 I iNKT cells and IL-2 with 5-fluorouracil Relapsed/advanced HCC, BCLC stage C
NCT03199807 Not yet 2017 IB/II Personalized new antigen reactive Advanced HCC, unresectable and no
recruiting immune cells (NRT), radiotherapy chemotherapy before
NCT03130712 Recruiting 2017 I/II GPC3-CAR T cells intratumor injection Advanced HCC, persistent cancer after at
least one prior standard of chemotherapy or
surgery
NCT03132792 Recruiting 2017 I Autologous genetically modified AFPᶜ³³²T Positive for HLA-A*02:01 or HLA-A*02:642
cells: genetically changed T cells that allele
target alpha-fetoprotein
NCT03302403 Not yet 2017 N/A Autologous T cells transduced with CAR Advanced HCC with previous ablation or
recruiting recognizing CD19, BCMA, GPC3 and resection in the last 4 to 12 weeks
Claudin18.2
NCT02905188 Not yet 2018 I GPC3-CAR T cells with fludarabine and BCLC stage A/B/C
recruiting cytoxan
NCT03441100 Not yet 2018 I IMA202 Product (CAR T cell) with HCC not amenable to treatments with
recruiting fludarabine and cyclophosphamide curative intent
HCC: hepatocellular carcinoma; CIK: cytokine induced killer; TACE: transarterial chemoembolization; BCLC: Barcelona clinic liver
cancer; NK: natural killer; TIL: tumor infiltrating lymphocyte; IL-2: interleukin-2; VEGFR: vascular endothelial growth factor receptor;
CAR: chimeric antigen receptor; PBL: peripheral blood lymphocyte; NKT: natural killer T; MUC1: mucin1; GPC3: glypican-3; AFP: alpha-
fetoprotein; HLA: human leukocyte antigen; BCMA: B-cell maturation antigen; N/A: not applicable
in the control group (77%, 57/74). Moreover, the PFS was significantly improved in the CIK treatment group
(P = 0.01). All of the adverse events (AEs) were grade I or II and self-limiting. AEs associated with treatment
were fever (47%), headache (4%), nausea (4%), dizziness (1%), itching (1%) and tachycardia (1%).