Page 70 - Read Online
P. 70
Lee et al. Hepatoma Res 2018;4:51 I http://dx.doi.org/10.20517/2394-5079.2018.78 Page 5 of 18
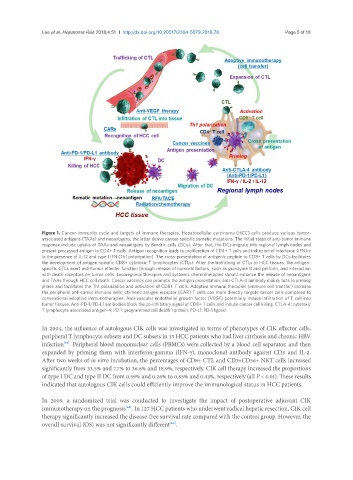
Figure 1. Cancer-immunity cycle and targets of immune therapies. Hepatocellular carcinoma (HCC) cells produce various tumor-
associated antigens (TAAs) and neoantigens; the latter derive cancer-specific somatic mutations. The initial steps of anti-tumor immune
response include uptake of TAAs and neoantigens by dendric cells (DCs). After that, the DCs migrate into regional lymph nodes and
present processed antigen to CD4+ T cells. Antigen recognition leads to proliferation of CD4+ T cells and induction of interferon (IFN)-γ
in the presence of IL-12 and type I IFN (Th1 polarization). The cross-presentation of antigenic peptide to CD8+ T cells by DCs facilitates
the development of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs). After the trafficking of CTLs to HCC tissues, the antigen-
specific CTLs exert anti-tumor effecter function through release of humoral factors, such as granzyme B and perforin, and interaction
with death receptors on tumor cells. Locoregional therapies and systemic chemotherapies should enhance the release of neoantigens
and TAAs through HCC cell death. Cancer vaccines can promote the antigen presentation; anti-CTLA-4 antibody mainly acts in priming
phase and facilitates the Th1 polarization and activation of CD8+ T cells. Adoptive immune therapies (immune cell transfer) increase
the peripheral anti-tumor immune cells; chimeric antigen receptor (CAR) T cells can more directly targets cancer cells compared to
conventional adoptive immunotherapies. Anti-vascular endothelial growth factor (VEGF) potentially induce infiltration of T cell into
tumor tissues. Anti-PD-1/PD-L1 antibodies block the co-inhibitory signal of CD8+ T cells and induce cancer cell killing. CTLA-4: cytotoxic
T lymphocyte associated antigen-4; PD-1: programmed cell death 1 protein; PD-L1: PD-1 ligand
In 2004, the influence of autologous CIK cells was investigated in terms of phenotypes of CIK effector cells,
peripheral T lymphocyte subsets and DC subsets in 13 HCC patients who had liver cirrhosis and chronic HBV
infection . Peripheral blood mononuclear cells (PBMCs) were collected by a blood cell separator, and then
[39]
expanded by priming them with interferon-gamma (IFN-γ), monoclonal antibody against CD3 and IL-2.
After two weeks of in vitro incubation, the percentages of CD8+ CTL and CD3+CD56+ NKT cells increased
significantly from 33.5% and 7.7% to 36.6% and 18.9%, respectively. CIK cell therapy increased the proportions
of type I DC and type II DC from 0.59% and 0.26% to 0.85% and 0.43%, respectively (all P < 0.01). These results
indicated that autologous CIK cells could efficiently improve the immunological status in HCC patients.
In 2009, a randomized trial was conducted to investigate the impact of postoperative adjuvant CIK
immunotherapy on the prognosis . In 127 HCC patients who underwent radical hepatic resection, CIK cell
[40]
therapy significantly increased the disease-free survival rate compared with the control group. However, the
overall survival (OS) was not significantly different .
[40]