Page 100 - Read Online
P. 100
Page 10 of 18 Karademir. Hepatoma Res 2018;4:58 I http://dx.doi.org/10.20517/2394-5079.2018.40
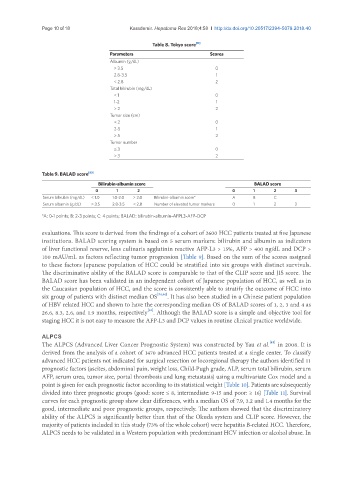
Table 8. Tokyo score [11]
Parameters Scores
Albumin (g/dL)
> 3.5 0
2.8-3.5 1
< 2.8 2
Total bilirubin (mg/dL)
< 1 0
1-2 1
> 2 2
Tumor size (cm)
< 2 0
2-5 1
> 5 2
Tumor number
≤ 3 0
> 3 2
Table 9. BALAD score [60]
Bilirubin-albumin score BALAD score
0 1 2 0 1 2 3
Serum bilirubin (mg/dL) < 1.0 1.0-2.0 > 2.0 Bilirubin-albumin score* A B C
Serum albumin (g/dL) > 3.5 2.8-3.5 < 2.8 Number of elevated tumor markers 0 1 2 3
*A: 0-1 points; B: 2-3 points; C: 4 points; BALAD: bilirubin-albumin-AFPL3-AFP-DCP
evaluations. This score is derived from the findings of a cohort of 2600 HCC patients treated at five Japanese
institutions. BALAD scoring system is based on 5 serum markers: bilirubin and albumin as indicators
of liver functional reserve, lens culinaris agglutinin reactive AFP-L3 > 15%, AFP > 400 ng/dL and DCP >
100 mAU/mL as factors reflecting tumor progression [Table 9]. Based on the sum of the scores assigned
to these factors Japanese population of HCC could be stratified into six groups with distinct survivals.
The discriminative ability of the BALAD score is comparable to that of the CLIP score and JIS score. The
BALAD score has been validated in an independent cohort of Japanese population of HCC, as well as in
the Caucasian population of HCC, and the score is consistently able to stratify the outcome of HCC into
six group of patients with distinct median OS [54,61] . It has also been studied in a Chinese patient population
of HBV related HCC and shown to have the corresponding median OS of BALAD scores of 1, 2, 3 and 4 as
[62]
26.6, 8.3, 2.6, and 1.9 months, respectively . Although the BALAD score is a simple and objective tool for
staging HCC it is not easy to measure the AFP-L3 and DCP values in routine clinical practice worldwide.
ALPCS
[48]
The ALPCS (Advanced Liver Cancer Prognostic System) was constructed by Yau et al. in 2008. It is
derived from the analysis of a cohort of 1470 advanced HCC patients treated at a single center. To classify
advanced HCC patients not indicated for surgical resection or locoregional therapy the authors identified 11
prognostic factors (ascites, abdominal pain, weight loss, Child-Pugh grade, ALP, serum total bilirubin, serum
AFP, serum urea, tumor size, portal thrombosis and lung metastasis) using a multivariate Cox model and a
point is given for each prognostic factor according to its statistical weight [Table 10]. Patients are subsequently
divided into three prognostic groups (good: score ≤ 8, intermediate: 9-15 and poor: ≥ 16) [Table 11]. Survival
curves for each prognostic group show clear differences, with a median OS of 7.9, 3.2 and 1.4 months for the
good, intermediate and poor prognostic groups, respectively. The authors showed that the discriminatory
ability of the ALPCS is significantly better than that of the Okuda system and CLIP score. However, the
majority of patients included in this study (73% of the whole cohort) were hepatitis B-related HCC. Therefore,
ALPCS needs to be validated in a Western population with predominant HCV infection or alcohol abuse. In