Page 189 - Read Online
P. 189
Costa et al. Hepatoma Res 2018;4:35 I http://dx.doi.org/10.20517/2394-5079.2018.06 Page 7 of 11
A 4.0 B 3.5
HCC pathologic classification 3.0 P = 0.01 HCC pathologic classification 3.0 P = 0.02
3.5
2.5
2.5
2
R = 0.44
2
R = 0.42
2.0
2.0
1.5 1.5
0 5 10 15 0 2 4 6 8
SUVmax SUVmax of tumor and liver ratio
C 4.0
HCC pathologic classification 3.0 P = 0.006
3.5
2.5
2
R = 0.54
2.0
1.5
0 5 10 15 20
SUVmax of tumor and muscle ratio
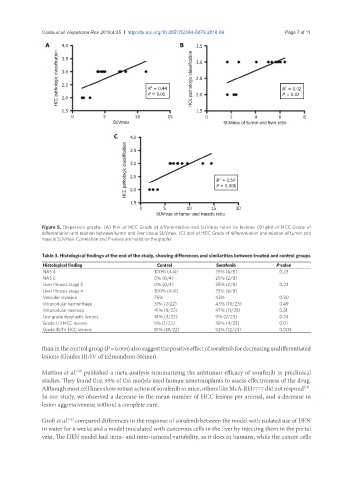
Figure 5. Dispersion graphs. (A) Plot of HCC Grade of differentiation and SUVmax taken by lesions; (B) plot of HCC Grade of
differentiation and relation between tumor and liver tissue SUVmax; (C) plot of HCC Grade of differentiation and relation of tumor and
muscle SUVmax. Correlation and P values are noted on the graphs
Table 3. Histological findings at the end of the study, showing differences and similarities between treated and control groups
Histological finding Control Sorafenib P value
NAS 4 100% (4/4) 75% (6/8) 0.23
NAS 6 0% (0/4) 25% (2/8)
Liver fibrosis stage 3 0% (0/4) 25% (2/8) 0.23
Liver fibrosis stage 4 100% (4/4) 75% (6/8)
Vascular invasion 75% 43% 0.30
Intranodular hemorrhage 31% (7/22) 43% (10/23) 0.49
Intranodular necrosis 41% (9/22) 47% (11/23) 0.51
Low grade dysplastic lesions 14% (3/22) 9% (2/23) 0.24
Grade I/II HCC lesions 5% (1/22) 39% (9/23) 0.01
Grade III/IV HCC lesions 81% (18/22) 52% (12/23) 0.003
than in the control group (P = 0.006) also suggest the positive effect of sorafenib for decreasing undifferentiated
lesions (Grades III/IV of Edmondson-Steiner).
Mattina et al. published a meta-analysis summarizing the antitumor efficacy of sorafenib in preclinical
[36]
studies. They found that 95% of the models used human xenotranplants to assess effectiveness of the drug.
Although most cell lines show robust action of sorafenib in mice, others like McA-RH7777 did not respond .
[12]
In our study, we observed a decrease in the mean number of HCC lesions per animal, and a decrease in
lesion aggressiveness; without a complete cure.
Groß et al. compared differences in the response of sorafenib between the model with isolated use of DEN
[12]
in water for 8 weeks and a model inoculated with cancerous cells in the liver by injecting them in the portal
vein. The DEN model had intra- and inter-tumoral variability, as it does in humans, while the cancer cells