Page 57 - Read Online
P. 57
Page 10 of 17 Chan et al. Hepatoma Res 2018;4:5 I http://dx.doi.org/10.20517/2394-5079.2017.49
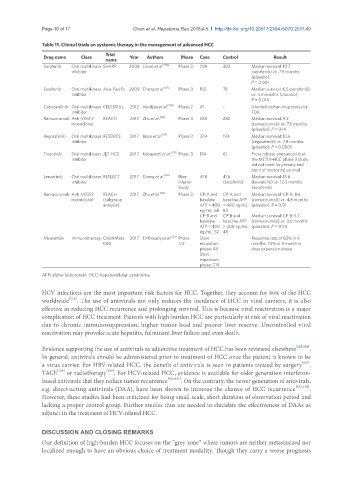
Table 11. Clinical trials on systemic therapy in the management of advanced HCC
Trial
Drug name Class Year Authors Phase Case Control Result
name
Sorafenib Oral multikinase SHARP 2008 Llovet et al. [110] Phase 3 299 303 Median survival: 10.7
inhibitor (sorafenib) vs. 7.9 months
(placebo);
P < 0.001
Sorafenib Oral multikinase Asia-Pacific 2009 Cheng et al. [111] Phase 3 150 76 Median survival: 6.5 (sorafenib)
inhibitor vs. 4.2 months (placebo);
P = 0.014
Cabozantinib Oral multikinase CELESTIAL 2012 Verslype et al. [134] Phase 2 41 - Granted orphan drug status by
inhibitor FDA
Ramucirumab Anti-VEGF2 REACH 2015 Zhu et al. [112] Phase 3 283 282 Median survival: 9.2
monoclonal (ramucirumab) vs. 7.6 months
(placebo); P = 0.14
Regorafenib Oral multikinase RESORCE 2017 Bruix et al. [117] Phase 3 379 193 Median survival: 10.6
inhibitor (regorafenib) vs. 7.8 months
(placebo); P < 0.0001
Tivantinib Oral multikinase JET-HCC 2017 Kobayashi et al. [135] Phase 3 134 61 Press release announced that
inhibitor the METIV-HCC phase 3 study
did not meet its primary end
point of improving survival
Lenvatinib Oral multikinase REFLECT 2017 Cheng et al. [116] Non- 478 476 Median survival: 13.6
inhibitor inferior (sorafenib) (lenvatinib) vs. 12.3 months
study (sorafenib)
Ramucirumab Anti-VEGF2 REACH 2017 Zhu et al. [112] Phase 3 CP-A and CP-A and Median survival: CP-A: 8.6
monoclonal (subgroup baseline baseline AFP (ramucirumab) vs. 4.8 months
analysis) AFP > 400 > 400 ng/mL: (placebo); P = 0.01
ng/mL: 68 83
CP-B and CP-B and Median survival: CP-B: 5.7
baseline baseline AFP (ramucirumab) vs. 3.6 months
AFP > 400 > 400 ng/mL: (placebo); P = 0.04
ng/mL: 52 48
Nivolumab Immunotherapy CheckMate 2017 El-Khoueiry et al. [123] Phase Dose Response rate of 83% in 6
040 1/2 escalation months; 74% in 9 month in
phase: 48 dose expansion phase
Dose-
expansion
phase: 214
AFP: alpha-fetoprotein; HCC: hepatocellular carcinoma
HCV infections are the most important risk factors for HCC. Together, they account for 80% of the HCC
worldwide [124] . The use of antivirals not only reduces the incidence of HCC in viral carriers, it is also
effective in reducing HCC recurrence and prolonging survival. This is because viral reactivation is a major
complication of HCC treatment. Patients with high-burden HCC are particularly at risk of viral reactivation
due to chronic immunosuppression, higher tumor load and poorer liver reserve. Uncontrolled viral
reactivation may provoke acute hepatitis, fulminant liver failure and even death.
Evidence supporting the use of antivirals as adjunctive treatment of HCC has been reviewed elsewhere [125,126] .
In general, antivirals should be administered prior to treatment of HCC once the patient is known to be
a virus carrier. For HBV-related HCC, the benefit of antivirals is seen in patients treated by surgery [127] ,
[129]
TACE [128] or radiotherapy . For HCV-related HCC, evidence is available for older generation interferon-
based antivirals that they reduce tumor recurrence [130,131] . On the contrary, the newer generation of antivirals,
e.g. direct-acting antivirals (DAA), have been shown to increase the chance of HCC recurrence [132,133] .
However, these studies had been criticized for being small scale, short duration of observation period and
lacking a proper control group. Further studies thus are needed to elucidate the effectiveness of DAAs as
adjunct in the treatment of HCV-related HCC.
DISCUSSION AND CLOSING REMARKS
Our definition of high-burden HCC focuses on the “grey zone” where tumors are neither metastasized nor
localized enough to have an obvious choice of treatment modality. Though they carry a worse prognosis