Page 54 - Read Online
P. 54
Chan et al. Hepatoma Res 2018;4:5 I http://dx.doi.org/10.20517/2394-5079.2017.49 Page 7 of 17
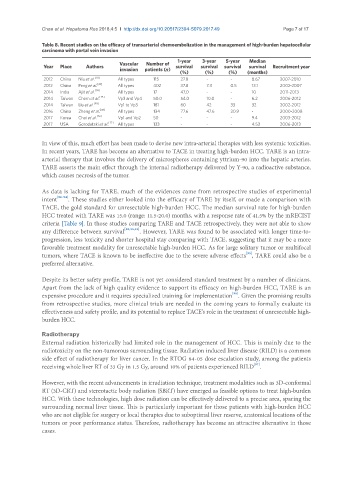
Table 8. Recent studies on the efficacy of transarterial chemoembolization in the management of high-burden hepatocellular
carcinoma with portal vein invasion
1-year 3-year 5-year Median
Vascular Number of
Year Place Authors survival survival survival survival Recruitment year
invasion patients (n)
(%) (%) (%) (months)
2012 China Niu et al. [78] All types 115 27.8 - - 8.67 2007-2010
2012 China Peng et al. [43] All types 402 37.8 7.3 0.5 13.1 2002-2007
2014 India Ajit et al. [74] All types 17 47.0 - - 10 2011-2013
2014 Taiwan Chern et al. [75] Vp3 and Vp4 50.0 54.0 10.0 - 6.2 2006-2012
2014 Taiwan Liu et al. [48] Vp1 to Vp3 181 60 42 33 32 2002-2012
2016 China Zheng et al. [49] All types 134 77.6 47.6 20.9 - 2000-2008
2017 Korea Choi et al. [76] Vp1 and Vp2 50 - - - 9.4 2003-2012
2017 USA Gorodetski et al. [77] All types 133 - - - 4.53 2006-2013
In view of this, much effort has been made to devise new intra-arterial therapies with less systemic toxicities.
In recent years, TARE has become an alternative to TACE in treating high-burden HCC. TARE is an intra-
arterial therapy that involves the delivery of microspheres containing yttrium-90 into the hepatic arteries.
TARE asserts the main effect through the internal radiotherapy delivered by Y-90, a radioactive substance,
which causes necrosis of the tumor.
As data is lacking for TARE, much of the evidences came from retrospective studies of experimental
intent [86-94] . These studies either looked into the efficacy of TARE by itself, or made a comparison with
TACE, the gold standard for unresectable high-burden HCC. The median survival rate for high-burden
HCC treated with TARE was 15.0 (range: 11.5-20.0) months, with a response rate of 41.5% by the mRECIST
criteria [Table 9]. In those studies comparing TARE and TACE retrospectively, they were not able to show
any difference between survival [88,93,94] . However, TARE was found to be associated with longer time-to-
progression, less toxicity and shorter hospital stay comparing with TACE, suggesting that it may be a more
favorable treatment modality for unresectable high-burden HCC. As for large solitary tumor or multifocal
tumors, where TACE is known to be ineffective due to the severe adverse effects , TARE could also be a
[95]
preferred alternative.
Despite its better safety profile, TARE is not yet considered standard treatment by a number of clinicians.
Apart from the lack of high quality evidence to support its efficacy on high-burden HCC, TARE is an
[96]
expensive procedure and it requires specialized training for implementation . Given the promising results
from retrospective studies, more clinical trials are needed in the coming years to formally evaluate its
effectiveness and safety profile, and its potential to replace TACE’s role in the treatment of unresectable high-
burden HCC.
Radiotherapy
External radiation historically had limited role in the management of HCC. This is mainly due to the
radiotoxicity on the non-tumorous surrounding tissue. Radiation induced liver disease (RILD) is a common
side effect of radiotherapy for liver cancer. In the RTOG 84-05 dose escalation study, among the patients
receiving whole liver RT of 33 Gy in 1.5 Gy, around 10% of patients experienced RILD .
[97]
However, with the recent advancements in irradiation technique, treatment modalities such as 3D-conformal
RT (3D-CRT) and stereotactic body radiation (SBRT) have emerged as feasible options to treat high-burden
HCC. With these technologies, high dose radiation can be effectively delivered to a precise area, sparing the
surrounding normal liver tissue. This is particularly important for those patients with high-burden HCC
who are not eligible for surgery or local therapies due to suboptimal liver reserve, anatomical locations of the
tumors or poor performance status. Therefore, radiotherapy has become an attractive alternative in those
cases.