Page 155 - Read Online
P. 155
Page 6 of 21 Yang et al. Hepatoma Res 2023;9:48 https://dx.doi.org/10.20517/2394-5079.2023.68
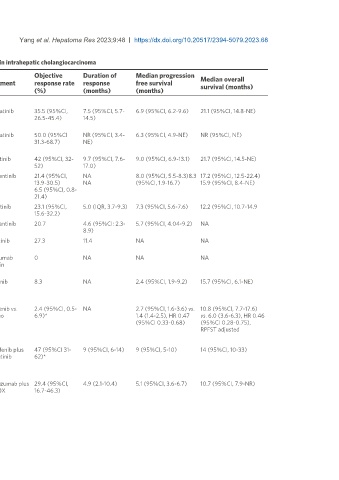
Table 2. Clinical trials evaluating targeted therapies against most frequent actionable alterations in intrahepatic cholangiocarcinoma
Objective Duration of Median progression
NO. of Median overall
Phase Trial Tumour type Treatment response rate response free survival
patients survival (months)
(%) (months) (months)
FGFR
Abou-Alfa et al. 2 FIGHT-202/NCT02924376 107 CCA (FGFR2 Pemigatinib 35.5 (95%CI, 7.5 (95%CI, 5.7- 6.9 (95%CI, 6.2-9.6) 21.1 (95%CI, 14.8-NE)
[28]
(2020) (105) fus) 26.5-45.4) 14.5)
(iCCA)
Shi et al. 2 NCT04256980 31 CCA (FGFR2 Pemigatinib 50.0 (95%CI NR (95%CI, 3.4- 6.3 (95%CI, 4.9-NE) NR (95%CI, NE)
[29]
(2023) (30) fus) 31.3-68.7) NE)
(iCCA)
Goyal et al. 2 FOENIX-CCA2/NCT02052778 103 iCCA (FGFR2 Futibatinib 42 (95%CI, 32- 9.7 (95%CI, 7.6- 9.0 (95%CI, 6.9-13.1) 21.7 (95%CI, 14.5-NE)
[7]
(2023) fus) 52) 17.0)
Borad et al. 2 FIDES-01/NCT03230318 103 iCCA (FGFR2 Derazantinib 21.4 (95%CI, NA 8.0 (95%CI, 5.5-8.3)8.3 17.2 (95%CI, 12.5-22.4)
[32]
(2022) 40 fus) 13.9-30.5) NA (95%CI, 1.9-16.7) 15.9 (95%CI, 8.4-NE)
iCCA (FGFR2 6.5 (95%CI, 0.8-
mut/amp) 21.4)
Javle et al. 2 NCT02150967 108 CCA (FGFR2 Infigratinib 23.1 (95%CI, 5.0 (IQR, 3.7-9.3) 7.3 (95%CI, 5.6-7.6) 12.2 (95%CI, 10.7-14.9
[31]
(2021) fus) 15.6-32.2)
Mazzaferro et 1/2 NCT01752920 29 iCCA (FGFR2 Derazantinib 20.7 4.6 (95%CI: 2.3- 5.7 (95%CI, 4.04-9.2) NA
[99]
al. (2019) fus) 8.9)
Bahleda et al. 1 NCT01703481 11 CCA (FGFR Erdafitinib 27.3 11.4 NA NA
[100]
(2019) mut/fus)
Kim et al. 1 NCT02368951 20 Solid Tumors Aprutumab 0 NA NA NA
[101]
(2019) (4) (FGFR2- ixadotin
positive) (CCA)
Ahn et al. pilot NCT02265341 12 BTCs (FGFR2 Ponatinib 8.3 NA 2.4 (95%CI, 1.9-9.2) 15.7 (95%CI, 6.1-NE)
[102]
(2022) (10) fus) (iCCA)
IDH
Abou-Alfa et al. 3 ClarIDHy/NCT02989857 185 iCCA (IDH1 Ivosidenib vs. 2.4 (95%CI, 0.5- NA 2.7 (95%CI, 1.6-3.6) vs. 10.8 (95%CI, 7.7-17.6)
[48]
(2020) mut) Placebo 6.9)* 1.4 (1.4-2.5), HR 0.47 vs. 6.0 (3.6-6.3), HR 0.46
(95%CI 0.33-0.68) (95%CI 0.28-0.75),
RPFST adjusted
BRAF
Subbiah et al. 2 ROAR/NCT02034110 43 BTCs (BRAF Dabrafenib plus 47 (95%CI 31- 9 (95%CI, 6-14) 9 (95%CI, 5-10) 14 (95%CI, 10-33)
[65]
(2020) (39) V600E) Trametinib 62)*
(iCCA)
HER2
Lee et al. 2 KCSG-HB19-14/NCT04722133 34 BTCs (HER2- Trastuzumab plus 29.4 (95%CI, 4.9 (2.1-10.4) 5.1 (95%CI, 3.6-6.7) 10.7 (95%CI, 7.9-NR)
[103]
(2023) (6) positive) FOLFOX 16.7-46.3)
(iCCA)