Page 85 - Read Online
P. 85
Page 179 Gupta et al. Extracell Vesicles Circ Nucleic Acids 2023;4:170-90 https://dx.doi.org/10.20517/evcna.2023.12
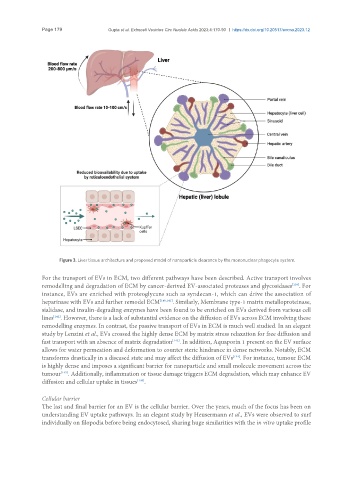
Figure 3. Liver tissue architecture and proposed model of nanoparticle clearance by the mononuclear phagocyte system.
For the transport of EVs in ECM, two different pathways have been described. Active transport involves
[139]
remodelling and degradation of ECM by cancer-derived EV-associated proteases and glycosidases . For
instance, EVs are enriched with proteoglycans such as syndecan-1, which can drive the association of
heparinase with EVs and further remodel ECM [140,141] . Similarly, Membrane type-1 matrix metalloproteinase,
sialidase, and insulin-degrading enzymes have been found to be enriched on EVs derived from various cell
lines . However, there is a lack of substantial evidence on the diffusion of EVs across ECM involving these
[142]
remodelling enzymes. In contrast, the passive transport of EVs in ECM is much well studied. In an elegant
study by Lenzini et al., EVs crossed the highly dense ECM by matrix stress relaxation for free diffusion and
fast transport with an absence of matrix degradation . In addition, Aquaporin 1 present on the EV surface
[143]
allows for water permeation and deformation to counter steric hindrance in dense networks. Notably, ECM
transforms drastically in a diseased state and may affect the diffusion of EVs . For instance, tumour ECM
[144]
is highly dense and imposes a significant barrier for nanoparticle and small molecule movement across the
[145]
tumour . Additionally, inflammation or tissue damage triggers ECM degradation, which may enhance EV
[146]
diffusion and cellular uptake in tissues .
Cellular barrier
The last and final barrier for an EV is the cellular barrier. Over the years, much of the focus has been on
understanding EV uptake pathways. In an elegant study by Heusermann et al., EVs were observed to surf
individually on filopodia before being endocytosed, sharing huge similarities with the in vitro uptake profile