Page 83 - Read Online
P. 83
Page 177 Gupta et al. Extracell Vesicles Circ Nucleic Acids 2023;4:170-90 https://dx.doi.org/10.20517/evcna.2023.12
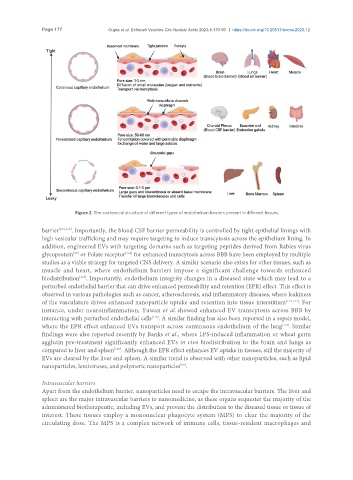
Figure 2. The anatomical structure of different types of endothelium barriers present in different tissues.
barrier [109,113] . Importantly, the blood CSF barrier permeability is controlled by tight epithelial linings with
high vesicular trafficking and may require targeting to induce transcytosis across the epithelium lining. In
addition, engineered EVs with targeting domains such as targeting peptides derived from Rabies virus
[114]
glycoprotein or Folate receptor for enhanced transcytosis across BBB have been employed by multiple
[93]
studies as a viable strategy for targeted CNS delivery. A similar scenario also exists for other tissues, such as
muscle and heart, where endothelium barriers impose a significant challenge towards enhanced
[107]
biodistribution . Importantly, endothelium integrity changes in a diseased state which may lead to a
perturbed endothelial barrier that can drive enhanced permeability and retention (EPR) effect. This effect is
observed in various pathologies such as cancer, atherosclerosis, and inflammatory diseases, where leakiness
of the vasculature drives enhanced nanoparticle uptake and retention into tissue interstitium [115-117] . For
instance, under neuroinflammation, Yuwan et al. showed enhanced EV transcytosis across BBB by
interacting with perturbed endothelial cells . A similar finding has also been reported in a sepsis model,
[118]
where the EPR effect enhanced EVs transport across continuous endothelium of the lung . Similar
[119]
findings were also reported recently by Banks et al., where LPS-induced inflammation or wheat germ
agglutin pre-treatment significantly enhanced EVs in vivo biodistribution to the brain and lungs as
[120]
compared to liver and spleen . Although the EPR effect enhances EV uptake in tissues, still the majority of
EVs are cleared by the liver and spleen. A similar trend is observed with other nanoparticles, such as lipid
nanoparticles, lentiviruses, and polymeric nanoparticles .
[99]
Intravascular barriers
Apart from the endothelium barrier, nanoparticles need to escape the intravascular barriers. The liver and
spleen are the major intravascular barriers to nanomedicine, as these organs sequester the majority of the
administered biotherapeutic, including EVs, and prevent the distribution to the diseased tissue or tissue of
interest. These tissues employ a mononuclear phagocyte system (MPS) to clear the majority of the
circulating dose. The MPS is a complex network of immune cells, tissue-resident macrophages and