Page 129 - Read Online
P. 129
Page 249 Raposo et al. Extracell Vesicles Circ Nucleic Acids 2023;4:240-54 https://dx.doi.org/10.20517/evcna.2023.18
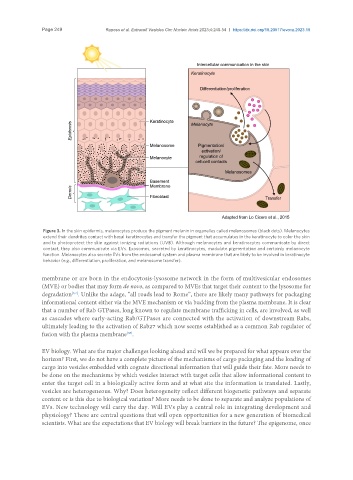
Figure 3. In the skin epidermis, melanocytes produce the pigment melanin in organelles called melanosomes (black dots). Melanocytes
extend their dendrites contact with basal keratinocytes and transfer the pigment that accumulates in the keratinocyte to color the skin
and to photoprotect the skin against ionizing radiations (UVB). Although melanocytes and keratinocytes communicate by direct
contact, they also communicate via EVs. Exosomes, secreted by keratinocytes, modulate pigmentation and certainly melanocyte
function. Melanocytes also secrete EVs from the endosomal system and plasma membrane that are likely to be involved in keratinocyte
behavior (e.g., differentiation, proliferation, and melanosome transfer).
membrane or are born in the endocytosis-lysosome network in the form of multivesicular endosomes
(MVE) or bodies that may form de novo, as compared to MVEs that target their content to the lysosome for
degradation . Unlike the adage, “all roads lead to Rome”, there are likely many pathways for packaging
[6,7]
informational content either via the MVE mechanism or via budding from the plasma membrane. It is clear
that a number of Rab GTPases, long known to regulate membrane trafficking in cells, are involved, as well
as cascades where early-acting Rab/GTPases are connected with the activation of downstream Rabs,
ultimately leading to the activation of Rab27 which now seems established as a common Rab regulator of
fusion with the plasma membrane .
[66]
EV biology. What are the major challenges looking ahead and will we be prepared for what appears over the
horizon? First, we do not have a complete picture of the mechanisms of cargo packaging and the loading of
cargo into vesicles embedded with cognate directional information that will guide their fate. More needs to
be done on the mechanisms by which vesicles interact with target cells that allow informational content to
enter the target cell in a biologically active form and at what site the information is translated. Lastly,
vesicles are heterogeneous. Why? Does heterogeneity reflect different biogenetic pathways and separate
content or is this due to biological variation? More needs to be done to separate and analyze populations of
EVs. New technology will carry the day. Will EVs play a central role in integrating development and
physiology? These are central questions that will open opportunities for a new generation of biomedical
scientists. What are the expectations that EV biology will break barriers in the future? The epigenome, once