Page 86 - Read Online
P. 86
Chen et al. Energy Mater. 2025, 5, 500045 https://dx.doi.org/10.20517/energymater.2024.144 Page 13 of 27
[103]
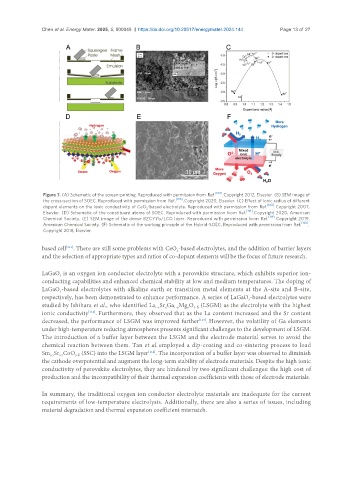
Figure 7. (A) Schematic of the screen-printing. Reproduced with permission from Ref. . Copyright 2012, Elsevier. (B) SEM image of
the cross-section of SOEC. Reproduced with permission from Ref. [106] . Copyright 2020, Elsevier. (C) Effect of ionic radius of different
dopant elements on the ionic conductivity of CeO -based electrolyte. Reproduced with permission from Ref. [108] . Copyright 2007,
2
Elsevier. (D) Schematic of the constituent atoms of SOEC. Reproduced with permission from Ref. [118] . Copyright 2020, American
Chemical Society. (E) SEM image of the dense BZCYYb/LCO layer. Reproduced with permission from Ref. [119] . Copyright 2019,
American Chemical Society. (F) Schematic of the working principle of the Hybrid-SOEC. Reproduced with permission from Ref. [120] .
Copyright 2018, Elsevier.
based cell . There are still some problems with CeO -based electrolytes, and the addition of barrier layers
[111]
2
and the selection of appropriate types and ratios of co-dopant elements will be the focus of future research.
LaGaO is an oxygen ion conductor electrolyte with a perovskite structure, which exhibits superior ion-
3
conducting capabilities and enhanced chemical stability at low and medium temperatures. The doping of
LaGaO -based electrolytes with alkaline earth or transition metal elements at the A-site and B-site,
3
respectively, has been demonstrated to enhance performance. A series of LaGaO -based electrolytes were
3
studied by Ishihara et al., who identified La Sr Ga Mg O (LSGM) as the electrolyte with the highest
1-y
1-x
x
3-δ
y
[112]
ionic conductivity . Furthermore, they observed that as the La content increased and the Sr content
[113]
decreased, the performance of LSGM was improved further . However, the volatility of Ga elements
under high-temperature reducing atmospheres presents significant challenges to the development of LSGM.
The introduction of a buffer layer between the LSGM and the electrode material serves to avoid the
chemical reaction between them. Tan et al. employed a dip-coating and co-sintering process to load
[114]
Sm Sr CoO (SSC) into the LSGM layer . The incorporation of a buffer layer was observed to diminish
0.5
0.5
3-δ
the cathode overpotential and augment the long-term stability of electrode materials. Despite the high ionic
conductivity of perovskite electrolytes, they are hindered by two significant challenges: the high cost of
production and the incompatibility of their thermal expansion coefficients with those of electrode materials.
In summary, the traditional oxygen ion conductor electrolyte materials are inadequate for the current
requirements of low-temperature electrolysis. Additionally, there are also a series of issues, including
material degradation and thermal expansion coefficient mismatch.