Page 11 - Read Online
P. 11
Page 6 of 11 Lv et al. Energy Mater 2024;4:400018 https://dx.doi.org/10.20517/energymater.2023.90
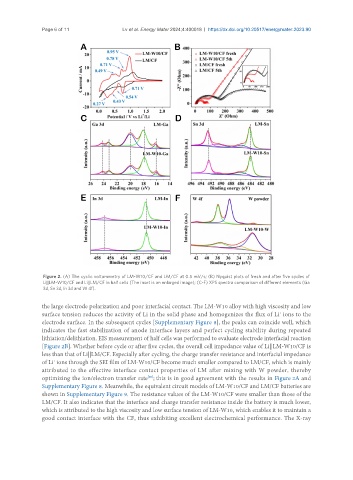
Figure 2. (A) The cyclic voltammetry of LM-W10/CF and LM/CF at 0.5 mV/s; (B) Nyquist plots of fresh and after five cycles of
Li||LM-W10/CF and Li||LM/CF in half cells (The inset is an enlarged image); (C-F) XPS spectra comparison of different elements (Ga
3d, Sn 3d, ln 3d and W 4f).
the large electrode polarization and poor interfacial contact. The LM-W10 alloy with high viscosity and low
surface tension reduces the activity of Li in the solid phase and homogenizes the flux of Li ions to the
+
electrode surface. In the subsequent cycles [Supplementary Figure 8], the peaks can coincide well, which
indicates the fast stabilization of anode interface layers and perfect cycling stability during repeated
lithiation/delithiation. EIS measurement of half cells was performed to evaluate electrode interfacial reaction
[Figure 2B]. Whether before cycle or after five cycles, the overall cell impedance value of Li||LM-W10/CF is
less than that of Li||LM/CF. Especially after cycling, the charge transfer resistance and interfacial impedance
of Li ions through the SEI film of LM-W10/CF become much smaller compared to LM/CF, which is mainly
+
attributed to the effective interface contact properties of LM after mixing with W powder, thereby
[20]
optimizing the ion/electron transfer rate ; this is in good agreement with the results in Figure 2A and
Supplementary Figure 8. Meanwhile, the equivalent circuit models of LM-W10/CF and LM/CF batteries are
shown in Supplementary Figure 9. The resistance values of the LM-W10/CF were smaller than those of the
LM/CF. It also indicates that the interface and charge transfer resistance inside the battery is much lower,
which is attributed to the high viscosity and low surface tension of LM-W10, which enables it to maintain a
good contact interface with the CF, thus exhibiting excellent electrochemical performance. The X-ray