Page 119 - Read Online
P. 119
Liang et al. Energy Mater 2023;3:300006 https://dx.doi.org/10.20517/energymater.2022.63 Page 7 of 14
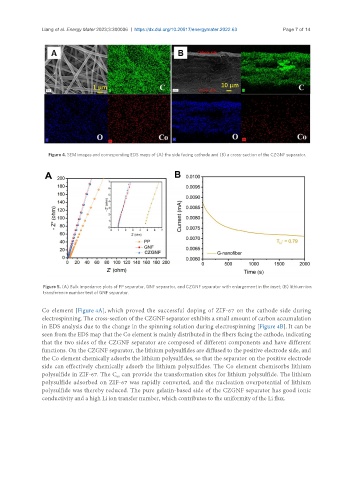
Figure 4. SEM images and corresponding EDS maps of (A) the side facing cathode and (B) a cross-section of the CZGNF separator.
Figure 5. (A) Bulk impedance plots of PP separator, GNF separator, and CZGNF separator with enlargement in the inset; (B) lithium-ion
transference number test of GNF separator.
Co element [Figure 4A], which proved the successful doping of ZIF-67 on the cathode side during
electrospinning. The cross-section of the CZGNF separator exhibits a small amount of carbon accumulation
in EDS analysis due to the change in the spinning solution during electrospinning [Figure 4B]. It can be
seen from the EDS map that the Co element is mainly distributed in the fibers facing the cathode, indicating
that the two sides of the CZGNF separator are composed of different components and have different
functions. On the CZGNF separator, the lithium polysulfides are diffused to the positive electrode side, and
the Co element chemically adsorbs the lithium polysulfides, so that the separator on the positive electrode
side can effectively chemically adsorb the lithium polysulfides. The Co element chemisorbs lithium
polysulfide in ZIF-67. The C can provide the transformation sites for lithium polysulfide. The lithium
60
polysulfide adsorbed on ZIF-67 was rapidly converted, and the nucleation overpotential of lithium
polysulfide was thereby reduced. The pure gelatin-based side of the CZGNF separator has good ionic
conductivity and a high Li ion transfer number, which contributes to the uniformity of the Li flux.