Page 117 - Read Online
P. 117
Liang et al. Energy Mater 2023;3:300006 https://dx.doi.org/10.20517/energymater.2022.63 Page 5 of 14
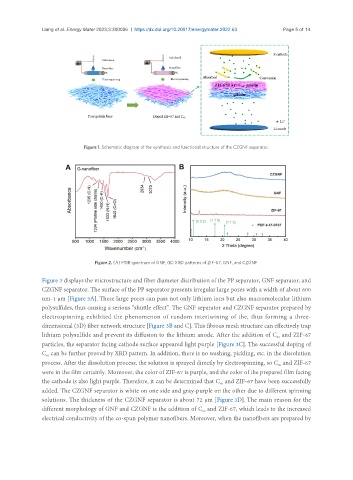
Figure 1. Schematic diagram of the synthesis and functional structure of the CZGNF separator.
Figure 2. (A) FTIR spectrum of GNF; (B) XRD patterns of ZIF-67, GNF, and CZGNF.
Figure 3 displays the microstructure and fiber diameter distribution of the PP separator, GNF separator, and
CZGNF separator. The surface of the PP separator presents irregular large pores with a width of about 600
nm-1 μm [Figure 3A]. These large pores can pass not only lithium ions but also macromolecular lithium
polysulfides, thus causing a serious “shuttle effect”. The GNF separator and CZGNF separator prepared by
electrospinning exhibited the phenomenon of random intertwining of the, thus forming a three-
dimensional (3D) fiber network structure [Figure 3B and C]. This fibrous mesh structure can effectively trap
lithium polysulfide and prevent its diffusion to the lithium anode. After the addition of C and ZIF-67
60
particles, the separator facing cathode surface appeared light purple [Figure 3C]. The successful doping of
C can be further proved by XRD pattern. In addition, there is no washing, pickling, etc. in the dissolution
60
process. After the dissolution process, the solution is sprayed directly by electrospinning, so C and ZIF-67
60
were in the film certainly. Moreover, the color of ZIF-67 is purple, and the color of the prepared film facing
the cathode is also light purple. Therefore, it can be determined that C and ZIF-67 have been successfully
60
added. The CZGNF separator is white on one side and gray-purple on the other due to different spinning
solutions. The thickness of the CZGNF separator is about 72 μm [Figure 3D]. The main reason for the
different morphology of GNF and CZGNF is the addition of C and ZIF-67, which leads to the increased
60
electrical conductivity of the co-spun polymer nanofibers. Moreover, when the nanofibers are prepared by