Page 92 - Read Online
P. 92
Page 2 of 9 Wang et al. Chem Synth 2023;3:9 https://dx.doi.org/10.20517/cs.2022.44
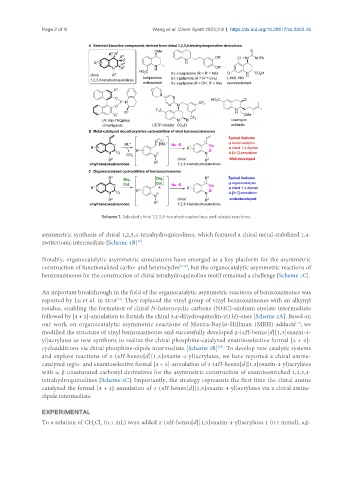
Scheme 1. Selected chiral 1,2,3,4-tetrahydroquinolines and related reactions.
asymmetric synthesis of chiral 1,2,3,4-tetrahydroquinolines, which featured a chiral metal-stabilized 1,4-
[8]
zwitterionic intermediate [Scheme 1B] .
Notably, organocatalytic asymmetric annulations have emerged as a key platform for the asymmetric
construction of functionalized carbo- and heterocycles [9-12] , but the organocatalytic asymmetric reactions of
benzoxazinones for the construction of chiral tetrahydroquinoline motif remained a challenge [Scheme 1C].
An important breakthrough in the field of the organocatalytic asymmetric reactions of benzoxazinones was
reported by Lu et al. in 2018 . They replaced the vinyl group of vinyl benzoxazinones with an alkynyl
[13]
residue, enabling the formation of chiral N-heterocyclic carbene (NHC)-azolium enolate intermediate
followed by [4 + 2]-annulation to furnish the chiral 3,4-dihydroquinolin-2(1H)-ones [Scheme 2A]. Based on
our work on organocatalytic asymmetric reactions of Morita-Baylis-Hillman (MBH) adducts , we
[14]
modified the structure of vinyl benzoxazinones and successfully developed 2-(4H-benzo[d][1,3]oxazin-4-
yl)acrylates as new synthons to realize the chiral phosphine-catalyzed enantioselective formal [4 + 2]-
cycloadditions via chiral phosphine-dipole intermediate [Scheme 2B] . To develop new catalytic systems
[15]
and explore reactions of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates, we here reported a chiral amine-
catalyzed regio- and enantioselective formal [4 + 2]-annulation of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates
with α, β-unsaturated carbonyl derivatives for the asymmetric construction of enantioenriched 1,2,3,4-
tetrahydroquinolines [Scheme 2C]. Importantly, the strategy represents the first time the chiral amine
catalyzed the formal [4 + 2]-annulation of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates via a chiral amine-
dipole intermediate.
EXPERIMENTAL
To a solution of CH Cl (0.1 mL) were added 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates 1 (0.1 mmol), α,β-
2
2