Page 97 - Read Online
P. 97
Wang et al. Chem Synth 2023;3:9 https://dx.doi.org/10.20517/cs.2022.44 Page 7 of 9
Scheme 5. Further investigations.
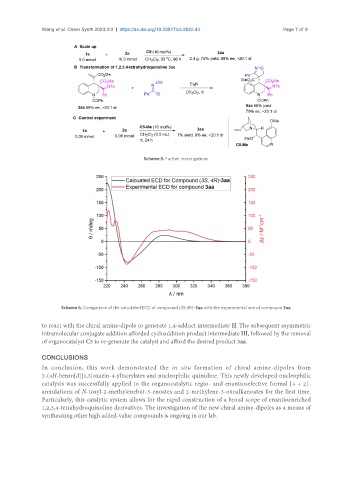
Scheme 6. Comparison of the calculated ECD of compound (3S,4R)-3aa with the experimental one of compound 3aa.
to react with the chiral amine-dipole to generate 1,4-adduct intermediate II. The subsequent asymmetric
intramolecular conjugate addition afforded cycloaddition product intermediate III, followed by the removal
of organocatalyst C5 to re-generate the catalyst and afford the desired product 3aa.
CONCLUSIONS
In conclusion, this work demonstrated the in situ formation of chiral amine-dipoles from
2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates and nucleophilic quinidine. This newly developed nucleophilic
catalysis was successfully applied to the organocatalytic regio- and enantioselective formal [4 + 2]-
annulations of N-tosyl-2-methylenebut-3-enoates and 2-methylene-3-oxoalkanoates for the first time.
Particularly, this catalytic system allows for the rapid construction of a broad scope of enantioenriched
1,2,3,4-tetrahydroquinoline derivatives. The investigation of the new chiral amine-dipoles as a means of
synthesizing other high added-value compounds is ongoing in our lab.