Page 147 - Read Online
P. 147
Page 4 of 9 Liu et al. Chem Synth 2023;3:22 https://dx.doi.org/10.20517/cs.2023.18
[a]
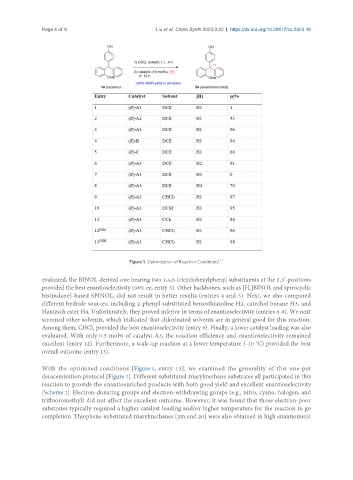
Figure 1. Optimization of Reaction Conditions .
evaluated, the BINOL-derived one bearing two 2,4,6-tricyclohexylphenyl substituents at the 3,3’-positions
provided the best enantioselectivity (96% ee, entry 3). Other backbones, such as [H ]BINOL and spirocyclic
8
bis(indane)-based SPINOL, did not result in better results (entries 4 and 5). Next, we also compared
different hydride sources, including 2-phenyl-substituted benzothiazoline H2, catechol borane H3, and
Hantzsch ester H4. Unfortunately, they proved inferior in terms of enantioselectivity (entries 6-8). We next
screened other solvents, which indicated that chlorinated solvents are in general good for this reaction.
Among them, CHCl provided the best enantioselectivity (entry 9). Finally, a lower catalyst loading was also
3
evaluated. With only 0.5 mol% of catalyst A3, the reaction efficiency and enantioselectivity remained
o
excellent (entry 12). Furthermore, a scale-up reaction at a lower temperature (-10 C) provided the best
overall outcome (entry 13).
With the optimized conditions [Figure 1, entry 13], we examined the generality of this one-pot
deracemization protocol [Figure 2]. Different substituted triarylmethane substrates all participated in this
reaction to provide the enantioenriched products with both good yield and excellent enantioselectivity
[Scheme 2]. Electron-donating groups and electron-withdrawing groups (e.g., nitro, cyano, halogen, and
trifluoromethyl) did not affect the excellent outcome. However, it was found that those electron-poor
substrates typically required a higher catalyst loading and/or higher temperature for the reaction to go
completion. Thiophene-substituted triarylmethanes [2m and 2o] were also obtained in high enantiomeric