Page 146 - Read Online
P. 146
Liu et al. Chem Synth 2023;3:22 https://dx.doi.org/10.20517/cs.2023.18 Page 3 of 9
3
2
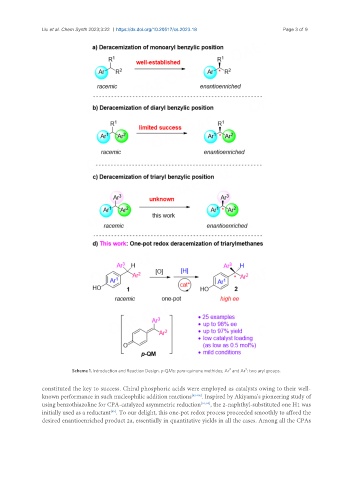
Scheme 1. Introduction and Reaction Design. p-QMs: para-quinone methides; Ar and Ar : two aryl groups.
constituted the key to success. Chiral phosphoric acids were employed as catalysts owing to their well-
known performance in such nucleophilic addition reactions [43-66] . Inspired by Akiyama’s pioneering study of
using benzothiazoline for CPA-catalyzed asymmetric reduction [57,58] , the 2-naphthyl-substituted one H1 was
[65]
initially used as a reductant . To our delight, this one-pot redox process proceeded smoothly to afford the
desired enantioenriched product 2a, essentially in quantitative yields in all the cases. Among all the CPAs