Page 46 - Read Online
P. 46
Bijnsdorp et al. Cancer Drug Resist 2021;4:719-27 https://dx.doi.org/10.20517/cdr.2021.21 Page 725
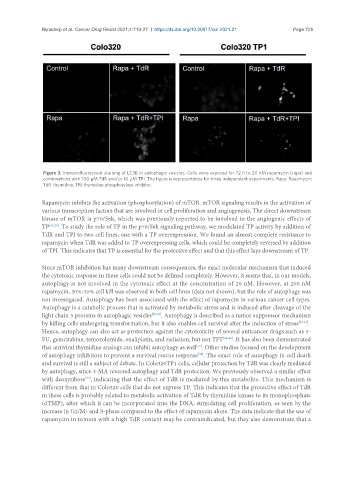
Figure 3. Immunofluorescent staining of LC3B in autophagic vesicles. Cells were exposed for 72 h to 20 nM rapamycin (rapa) and
combinations with 100 µM TdR and/or 10 µM TPI. The figure is representative for three independent experiments. Rapa: Rapamycin;
TdR: thymidine; TPI: thymidine phosphorylase inhibitor.
Rapamycin inhibits the activation (phosphorylation) of mTOR. mTOR signaling results in the activation of
various transcription factors that are involved in cell proliferation and angiogenesis. The direct downstream
kinase of mTOR is p70/S6k, which was previously reported to be involved in the angiogenic effects of
TP [11,20] . To study the role of TP in the p70/S6k signaling pathway, we modulated TP activity by addition of
TdR and TPI to two cell lines, one with a TP overexpression. We found an almost complete resistance to
rapamycin when TdR was added to TP overexpressing cells, which could be completely reversed by addition
of TPI. This indicates that TP is essential for the protective effect and that this effect lays downstream of TP.
Since mTOR inhibition has many downstream consequences, the exact molecular mechanism that induced
the cytotoxic response in these cells could not be defined completely. However, it seems that, in our models,
autophagy is not involved in the cytotoxic effect at the concentration of 20 nM. However, at 200 nM
rapamycin, 30%-35% cell kill was observed in both cell lines (data not shown), but the role of autophagy was
not investigated. Autophagy has been associated with the effect of rapamycin in various cancer cell types.
Autophagy is a catabolic process that is activated by metabolic stress and is induced after cleavage of the
light chain 3 proteins in autophagic vesicles [23,36] . Autophagy is described as a tumor suppressor mechanism
by killing cells undergoing transformation, but it also enables cell survival after the induction of stress [23,36] .
Hence, autophagy can also act as protection against the cytotoxicity of several anticancer drugs such as 5-
FU, gemcitabine, temozolomide, oxaliplatin, and radiation, but not TFT [24-28] . It has also been demonstrated
[37]
that antiviral thymidine analogs can inhibit autophagy as well . Other studies focused on the development
[38]
of autophagy inhibitors to prevent a survival rescue response . The exact role of autophagy in cell death
and survival is still a subject of debate. In Colo320TP1 cells, cellular protection by TdR was clearly mediated
by autophagy, since 3-MA reversed autophagy and TdR protection. We previously observed a similar effect
with deoxyribose , indicating that the effect of TdR is mediated by this metabolite. This mechanism is
[39]
different from that in Colo320 cells that do not express TP. This indicates that the protective effect of TdR
in these cells is probably related to metabolic activation of TdR by thymidine kinase to its monophosphate
(dTMP), after which it can be incorporated into the DNA, stimulating cell proliferation, as seen by the
increase in G2/M- and S-phase compared to the effect of rapamycin alone. The data indicate that the use of
rapamycin in tumors with a high TdR content may be contraindicated, but they also demonstrate that a