Page 51 - Read Online
P. 51
Page 368 Sale et al. Cancer Drug Resist 2019;2:365-80 I http://dx.doi.org/10.20517/cdr.2019.14
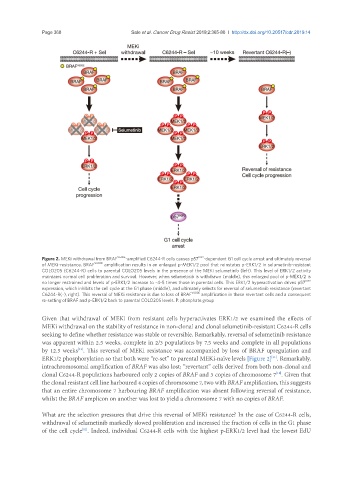
Figure 2. MEKi withdrawal from BRAF V600E -amplified C6244-R cells causes p57 KIP2 -dependent G1 cell cycle arrest and ultimately reversal
of MEKi-resistance. BRAF V600E amplification results in an enlarged p-MEK1/2 pool that reinstates p-ERK1/2 in selumetinib-resistant
COLO205 (C6244-R) cells to parental COLO205 levels in the presence of the MEKi selumetinib (left). This level of ERK1/2 activity
maintains normal cell proliferation and survival. However, when selumetinib is withdrawn (middle), this enlarged pool of p-MEK1/2 is
no longer restrained and levels of p-ERK1/2 increase to ~4-5 times those in parental cells. This ERK1/2 hyperactivation drives p57 KIP2
expression, which inhibits the cell cycle at the G1 phase (middle), and ultimately selects for reversal of selumetinib resistance (revertant
C6244-R(-), right). This reversal of MEKi resistance is due to loss of BRAF V600E amplification in these revertant cells and a consequent
re-setting of BRAF and p-ERK1/2 back to parental COLO205 levels. P: phosphate group
Given that withdrawal of MEKi from resistant cells hyperactivates ERK1/2 we examined the effects of
MEKi withdrawal on the stability of resistance in non-clonal and clonal selumetinib-resistant C6244-R cells
seeking to define whether resistance was stable or reversible. Remarkably, reversal of selumetinib resistance
was apparent within 2.5 weeks, complete in 2/3 populations by 7.5 weeks and complete in all populations
by 12.5 weeks . This reversal of MEKi resistance was accompanied by loss of BRAF upregulation and
[11]
ERK1/2 phosphorylation so that both were “re-set” to parental MEKi-naïve levels [Figure 2] . Remarkably,
[11]
intrachromosomal amplification of BRAF was also lost; “revertant” cells derived from both non-clonal and
clonal C6244-R populations harboured only 2 copies of BRAF and 3 copies of chromosome 7 . Given that
[11]
the clonal resistant cell line harboured 4 copies of chromosome 7, two with BRAF amplification, this suggests
that an entire chromosome 7 harbouring BRAF amplification was absent following reversal of resistance,
whilst the BRAF amplicon on another was lost to yield a chromosome 7 with no copies of BRAF.
What are the selection pressures that drive this reversal of MEKi resistance? In the case of C6244-R cells,
withdrawal of selumetinib markedly slowed proliferation and increased the fraction of cells in the G1 phase
of the cell cycle . Indeed, individual C6244-R cells with the highest p-ERK1/2 level had the lowest EdU
[11]