Page 48 - Read Online
P. 48
Page 226 Crisafulli et al. Cancer Drug Resist 2019;2:225-41 I http://dx.doi.org/10.20517/cdr.2018.008
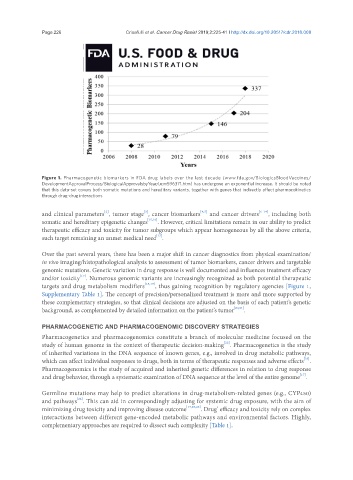
Figure 1. Pharmacogenetic biomarkers in FDA drug labels over the last decade (www.fda.gov/BiologicsBloodVaccines/
DevelopmentApprovalProcess/BiologicalApprovalsbyYear/ucm596371.htm) has undergone an exponential increase. It should be noted
that this data-set covers both somatic mutations and hereditary variants, together with genes that indirectly affect pharmacokinetics
through drug-drug interactions
[3]
[2]
[4,5]
and clinical parameters , tumor stage , cancer biomarkers and cancer drivers [6-14] , including both
somatic and hereditary epigenetic changes [15,16] . However, critical limitations remain in our ability to predict
therapeutic efficacy and toxicity for tumor subgroups which appear homogeneous by all the above criteria,
[17]
such target remaining an unmet medical need .
Over the past several years, there has been a major shift in cancer diagnostics from physical examination/
in vivo imaging/histopathological analysis to assessment of tumor biomarkers, cancer drivers and targetable
genomic mutations. Genetic variation in drug response is well documented and influences treatment efficacy
and/or toxicity . Numerous genomic variants are increasingly recognized as both potential therapeutic
[17]
targets and drug metabolism modifiers [18,19] , thus gaining recognition by regulatory agencies [Figure 1,
Supplementary Table 1]. The concept of precision/personalized treatment is more and more supported by
these complementary strategies, so that clinical decisions are adjusted on the basis of each patient’s genetic
background, as complemented by detailed information on the patient’s tumor [20,21] .
PHARMACOGENETIC AND PHARMACOGENOMIC DISCOVERY STRATEGIES
Pharmacogenetics and pharmacogenomics constitute a branch of molecular medicine focused on the
[22]
study of human genome in the context of therapeutic decision-making . Pharmacogenetics is the study
of inherited variations in the DNA sequence of known genes, e.g., involved in drug metabolic pathways,
[23]
which can affect individual responses to drugs, both in terms of therapeutic responses and adverse effects .
Pharmacogenomics is the study of acquired and inherited genetic differences in relation to drug response
[17]
and drug behavior, through a systematic examination of DNA sequence at the level of the entire genome .
Germline mutations may help to predict alterations in drug-metabolism-related genes (e.g., CYP450)
and pathways . This can aid in correspondingly adjusting for systemic drug exposure, with the aim of
[24]
minimizing drug toxicity and improving disease outcome [17,25,26] . Drug’ efficacy and toxicity rely on complex
interactions between different gene-encoded metabolic pathways and environmental factors. Highly,
complementary approaches are required to dissect such complexity [Table 1].