Page 204 - Read Online
P. 204
Page 700 Alonso-Peña et al. Cancer Drug Resist 2019;2:680-709 I http://dx.doi.org/10.20517/cdr.2019.006
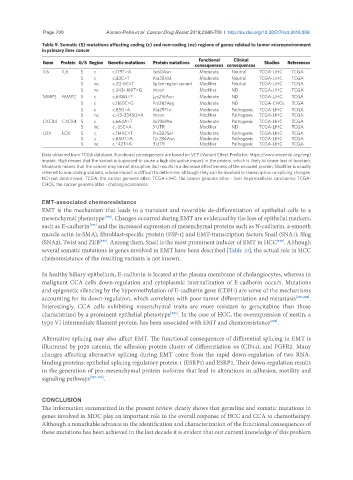
Table 9. Somatic (S) mutations affecting coding (c) and non-coding (nc) regions of genes related to tumor microenvironment
in primary liver cancer
Gene Protein G/S Region Genetic mutations Protein mutations Functional Clinical Studies References
consequences consequences
IL6 IL6 S c c.179T>A Ile60Asn Moderate Neutral TCGA-LIHC TCGA
S c c.83C>T Ala28Val Moderate Neutral TCGA-LIHC TCGA
S nc c.20-6C>T Splice region variant Modifier Neutral TCGA-LIHC TCGA
S nc c.243+169T>G Intron Modifier ND TCGA-LIHC TCGA
MMP2 MMP2 S c c.648G>T Lys216Asn Moderate ND TCGA-LIHC TCGA
S c c.1160C>G Pro387Arg Moderate ND TCGA-CHOL TCGA
S c c.85G>A Ala29Thr Moderate Pathogenic TCGA-LIHC TCGA
S nc c.-75-3345G>A Intron Modifier Pathogenic TCGA-LIHC TCGA
CXCR4 CXCR4 S c c.664A>T Ile226Phe Moderate Pathogenic TCGA-LIHC TCGA
S nc c.-55C>A 5’UTR Modifier ND TCGA-LIHC TCGA
LOX LOX S c c.1144C>T Pro382Ser Moderate Pathogenic TCGA-LIHC TCGA
S c c.850T>A Tyr284Asn Moderate Pathogenic TCGA-LIHC TCGA
S nc c.*42T>A 3’UTR Modifier Pathogenic TCGA-LIHC TCGA
Data obtained from TCGA database. Functional consequences are based on VEP (Variant Effect Predictor; https://www.ensembl.org/vep)
impact: High means that the variant is supposed to cause a high disruptive impact in the protein, which is likely to cause loss of function;
Moderate means that the variant may be not disruptive, but results in a decrease effectiveness of the encoded protein; Modifier is usually
referred to non-coding variants, whose impact is difficult to determine, although they can be involved in transcription or splicing changes.
ND: not determined; TCGA: the cancer genome atlas; TCGA-LIHC: the cancer genome atlas - liver hepatocellular carcinoma; TCGA-
CHOL: the cancer genome atlas - cholangiocarcinoma
EMT-associated chemoresistance
EMT is the mechanism that leads to a transient and reversible de-differentiation of epithelial cells to a
[196]
mesenchymal phenotype . Changes occurred during EMT are evidenced by the loss of epithelial markers,
such as E-cadherin and the increased expression of mesenchymal proteins such as N-cadherin, a-smooth
[197]
muscle actin (a-SMA), fibroblast-specific protein (FSP-1) and EMT-transcription factors Snail (SNA1), Slug
(SNA2), Twist and ZEB . Among them, Snail is the most prominent inducer of EMT in HCC . Although
[198]
[196]
several somatic mutations in genes involved in EMT have been described [Table 10], the actual role in HCC
chemoresistance of the resulting variants is not known.
In healthy biliary epithelium, E-cadherin is located at the plasma membrane of cholangiocytes, whereas in
malignant CCA cells down-regulation and cytoplasmic internalization of E-cadherin occurs. Mutations
and epigenetic silencing by the hypermethylation of E-cadherin gene (CDH1) are some of the mechanisms
accounting for its down-regulation, which correlates with poor tumor differentiation and metastasis [199-204] .
Interestingly, CCA cells exhibiting mesenchymal traits are more resistant to gemcitabine than those
characterized by a prominent epithelial phenotype . In the case of HCC, the overexpression of nestin, a
[205]
type VI intermediate filament protein, has been associated with EMT and chemoresistance [206] .
Alternative splicing may also affect EMT. The functional consequences of differential splicing in EMT is
illustrated by p120 catenin, the adhesion protein cluster of differentiation 44 (CD44), and FGFR2. Many
changes affecting alternative splicing during EMT come from the rapid down-regulation of two RNA-
binding proteins: epithelial splicing regulatory protein 1 (ESRP1) and ESRP2. Their down-regulation results
in the generation of pro-mesenchymal protein isoforms that lead to alterations in adhesion, motility and
signaling pathways [207-209] .
CONCLUSION
The information summarized in the present review clearly shows that germline and somatic mutations in
genes involved in MOC play an important role in the overall response of HCC and CCA to chemotherapy.
Although a remarkable advance in the identification and characterization of the functional consequences of
these mutations has been achieved in the last decade it is evident that our current knowledge of this problem