Page 124 - Read Online
P. 124
Page 779 Wong et al. Cancer Drug Resist 2023;6:768-87 https://dx.doi.org/10.20517/cdr.2023.58
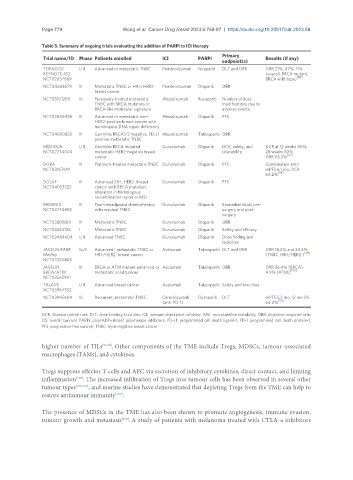
Table 3. Summary of ongoing trials evaluating the addition of PARPi to ICI therapy
Primary
Trial name/ID Phase Patients enrolled ICI PARPi Results (if any)
endpoint(s)
TOPACIO/ I/II Advanced or metastatic TNBC Pembrolizumab Niraparib DLT and ORR ORR 21%, 47%, 11%
KEYNOTE-162 (overall, BRCA mutant,
[96]
NCT02657889 BRCA wild-type)
NCT04683679 II Metastatic TNBC or HR+/HER2- Pembrolizumab Olaparib ORR
breast cancer
NCT03101280 Ib Previously treated metastatic Atezolizumab Rucaparib Number of dose
TNBC with BRCA mutation or modifications due to
BRCA-like molecular signature adverse events
NCT02849496 II Advanced or metastatic non- Atezolizumab Olaparib PFS
HER2-positive breast cancer with
homologous DNA repair deficiency
NCT04690855 II Germline BRCA1/2 negative, PD-L1 Atezolizumab Talazoparib ORR
positive metastatic TNBC
MEDIOLA I/II Germline BRCA mutated Durvalumab Olaparib DCR, safety, and DCR at 12 weeks 80%,
NCT02734004 metastatic HER2-negative breast tolerability 28 weeks 50%
[97]
cancer ORR 63.3%
DORA II Platinum-treated metastatic TNBC Durvalumab Olaparib PFS Combination arm:
NCT03167619 mPFS 6.1 mo, DCR
[98]
68.2%
DOLAF II Advanced ER+, HER2- breast Durvalumab Olaparib PFS
NCT04053322 cancer with BRCA mutation,
alteration in homologous
recombination repair or MSI
PHOENIX II Post-neoadjuvant chemotherapy Durvalumab Olaparib Biomarker study pre-
NCT03740893 with residual TNBC surgery and post-
surgery
NCT03801369 II Metastatic TNBC Durvalumab Olaparib ORR
NCT03544125 I Metastatic TNBC Durvalumab Olaparib Safety and efficacy
NCT02484404 I/II Advanced TNBC Durvalumab Olaparib Dose finding and
toxicities
JAVELIN PARP Ib/II Advanced/ metastatic TNBC or Avelumab Talazoparib DLT and ORR ORR 18.2% and 34.8%
Medley HR+/HER2- breast cancer (TNBC, HR+/HER2-) [99]
NCT03330405
JAVELIN II BRCA or ATM mutant advanced or Avelumab Talazoparib ORR ORR 26.4% (BRCA)
BRCA/ATM metastatic solid tumour 4.9% (ATM) [100]
NCT03565991
TALAVE I/II Advanced breast cancer Avelumab Talazoparib Safety and toxicities
NCT03964532
NCT03945604 Ib Recurrent, metastatic TNBC Camrelizumab Fluzoparib DLT mPFS 5.2 mo, 12 mo OS
[101]
(anti-PD-1) 64.2%
DCR: Disease control rate; DLT: dose-limiting toxicities; ICI: immune checkpoint inhibitor; MSI: microsatellite instability; ORR: objective response rate;
OS: overall survival; PARPi: poly(ADP-ribose) polymerase inhibitors; PD-L1: programmed cell death ligand-1; PD-1: programmed cell death protein-1;
PFS: progression-free survival; TNBC: triple-negative breast cancer.
higher number of TILs [51,52] . Other components of the TME include Tregs, MDSCs, tumour-associated
macrophages (TAMs), and cytokines.
Tregs suppress effector T cells and APC via secretion of inhibitory cytokines, direct contact, and limiting
[108]
inflammation . The increased infiltration of Tregs into tumour cells has been observed in several other
tumour types [109,110] , and murine studies have demonstrated that depleting Tregs from the TME can help to
restore antitumour immunity .
[109]
The presence of MDSCs in the TME has also been shown to promote angiogenesis, immune evasion,
tumour growth and metastasis . A study of patients with melanoma treated with CTLA-4 inhibitors
[108]