Page 123 - Read Online
P. 123
Wong et al. Cancer Drug Resist 2023;6:768-87 https://dx.doi.org/10.20517/cdr.2023.58 Page 778
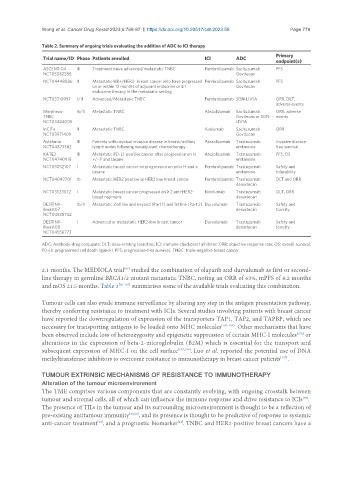
Table 2. Summary of ongoing trials evaluating the addition of ADC to ICI therapy
Primary
Trial name/ID Phase Patients enrolled ICI ADC
endpoint(s)
ASCENT-04 III Treatment naïve advanced/metastatic TNBC Pembrolizumab Sacituzumab PFS
NCT05382286 Govitecan
NCT04448886 II Metastatic HR+/HER2- breast cancer who have progressed Pembrolizumab Sacituzumab PFS
on or within 12 months of adjuvant endocrine or ≥ 1 Govitecan
endocrine therapy in the metastatic setting
NCT03310957 I/II Advanced/Metastatic TNBC Pembrolizumab SGN-LIV1A ORR, DLT,
adverse events
Morpheus- Ib/II Metastatic TNBC Atezolizumab Sacituzumab ORR, adverse
TNBC Govitecan or SGN- events
NCT03424005 LIV1A
InCITe II Metastatic TNBC Avelumab Sacituzumab ORR
NCT03971409 Govitecan
Astefania III Patients with residual invasive disease in breast/axillary Atezolizumab Trastuzumab Invasive disease-
NCT04873362 lymph nodes following neoadjuvant chemotherapy emtansine free survival
KATE3 III Metastatic PD-L1-positive cancer after progression on H Atezolizumab Trastuzumab PFS, OS
NCT04740918 +/- P and taxane emtansine
NCT03032107 I Metastatic breast cancer on progression on prior H and a Pembrolizumab Trastuzumab Safety and
taxane emtansine tolerability
NCT04042701 Ib Metastatic HER2 positive or HER2 low breast cancer Pembrolizumab Trastuzumab DLT and ORR
deruxtecan
NCT03523572 I Metastatic breast cancer progressed on ≥ 2 anti-HER2- Nivolumab Trastuzumab DLT, ORR
based regimens deruxtecan
DESTINY- Ib/II Metastatic 2nd line and beyond (Part 1) and 1st line (Part 2) Durvalumab Trastuzumab Safety and
Breast07 deruxtecan toxicity
NCT04538742
DESTINY- I Advanced or metastatic HER2-low breast cancer Durvalumab Trastuzumab Safety and
Breast08 deruxtecan toxicity
NCT04556773
ADC: Antibody-drug conjugate; DLT: dose-limiting toxicities; ICI: immune checkpoint inhibitor; ORR: objective response rate; OS: overall survival;
PD-L1: programmed cell death ligand-1; PFS: progression-free survival; TNBC: triple-negative breast cancer.
[97]
2.1 months. The MEDIOLA trial studied the combination of olaparib and durvalumab as first or second-
line therapy in germline BRCA1/2 mutant metastatic TNBC, noting an ORR of 63%, mPFS of 8.2 months
and mOS 21.5 months. Table 3 [96-101] summarises some of the available trials evaluating this combination.
Tumour cells can also evade immune surveillance by altering any step in the antigen presentation pathway,
thereby conferring resistance to treatment with ICIs. Several studies involving patients with breast cancer
have reported the downregulation of expression of the transporters TAP1, TAP2, and TAPBP, which are
necessary for transporting antigens to be loaded onto MHC molecules [102-104] . Other mechanisms that have
been observed include loss of heterozygosity and epigenetic suppression of certain MHC-I molecules or
[105]
alterations in the expression of beta-2-microglobulin (B2M) which is essential for the transport and
subsequent expression of MHC-I on the cell surface [105,106] . Luo et al. reported the potential use of DNA
methyltransferase inhibitors to overcome resistance to immunotherapy in breast cancer patients .
[107]
TUMOUR EXTRINSIC MECHANISMS OF RESISTANCE TO IMMUNOTHERAPY
Alteration of the tumour microenvironment
The TME comprises various components that are constantly evolving, with ongoing crosstalk between
tumour and stromal cells, all of which can influence the immune response and drive resistance to ICIs .
[73]
The presence of TILs in the tumour and its surrounding microenvironment is thought to be a reflection of
pre-existing antitumour immunity [49,50] , and its presence is thought to be predictive of response to systemic
[24]
[50]
anti-cancer treatment , and a prognostic biomarker . TNBC and HER2-positive breast cancers have a