Page 25 - Read Online
P. 25
Li et al. Ageing Neur Dis 2022;2:13 https://dx.doi.org/10.20517/and.2022.13 Page 3 of 13
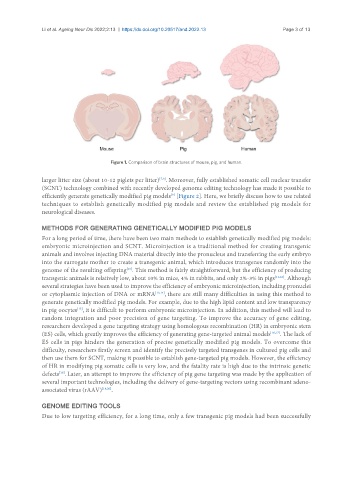
Figure 1. Comparison of brain structures of mouse, pig, and human.
[7,8]
larger litter size (about 10-12 piglets per litter) . Moreover, fully established somatic cell nuclear transfer
(SCNT) technology combined with recently developed genome editing technology has made it possible to
efficiently generate genetically modified pig models [Figure 2]. Here, we briefly discuss how to use related
[9]
techniques to establish genetically modified pig models and review the established pig models for
neurological diseases.
METHODS FOR GENERATING GENETICALLY MODIFIED PIG MODELS
For a long period of time, there have been two main methods to establish genetically modified pig models:
embryonic microinjection and SCNT. Microinjection is a traditional method for creating transgenic
animals and involves injecting DNA material directly into the pronucleus and transferring the early embryo
into the surrogate mother to create a transgenic animal, which introduces transgenes randomly into the
genome of the resulting offspring . This method is fairly straightforward, but the efficiency of producing
[10]
transgenic animals is relatively low, about 10% in mice, 4% in rabbits, and only 2%-3% in pigs [11,12] . Although
several strategies have been used to improve the efficiency of embryonic microinjection, including pronuclei
or cytoplasmic injection of DNA or mRNA [13,14] , there are still many difficulties in using this method to
generate genetically modified pig models. For example, due to the high lipid content and low transparency
in pig oocytes , it is difficult to perform embryonic microinjection. In addition, this method will lead to
[15]
random integration and poor precision of gene targeting. To improve the accuracy of gene editing,
researchers developed a gene targeting strategy using homologous recombination (HR) in embryonic stem
(ES) cells, which greatly improves the efficiency of generating gene-targeted animal models [16,17] . The lack of
ES cells in pigs hinders the generation of precise genetically modified pig models. To overcome this
difficulty, researchers firstly screen and identify the precisely targeted transgenes in cultured pig cells and
then use them for SCNT, making it possible to establish gene-targeted pig models. However, the efficiency
of HR in modifying pig somatic cells is very low, and the fatality rate is high due to the intrinsic genetic
defects . Later, an attempt to improve the efficiency of pig gene targeting was made by the application of
[18]
several important technologies, including the delivery of gene-targeting vectors using recombinant adeno-
associated virus (rAAV) [19,20] .
GENOME EDITING TOOLS
Due to low targeting efficiency, for a long time, only a few transgenic pig models had been successfully