Page 50 - Read Online
P. 50
Page 6 of 21 Persico et al. Rare Dis Orphan Drugs J 2023;2:xx https://dx.doi.org/10.20517/rdodj.2023.08
Table 1. Composition of the active compound. The active comparator is devoid of CoQ10 and contains all the remaining components
Daily dose by patient weight
Compounds
≤ 20 kg > 20 kg
CoQ10 50 mg 100 mg
Vitamin E 30 mg 60 mg
Vitamin B1(thiamine) 1.05 mg 2.10 mg
Vitamin B2 (riboflavin HCI) 1.2 mg 2.4 mg
Vitamin B3 (Niacin) 9 mg 18 mg
Vitamin B5 (Pantothenic acid) 4.5 mg 9 mg
Vitamin B6 (Pyridoxine) 1.5 mg 3 mg
Vitamin B12 (Cyanocobalamine) 4.5 μg 9 μg
Folic acid 0.1 mg 0.2 mg
Biotin 0.1125 mg 0.225 mg
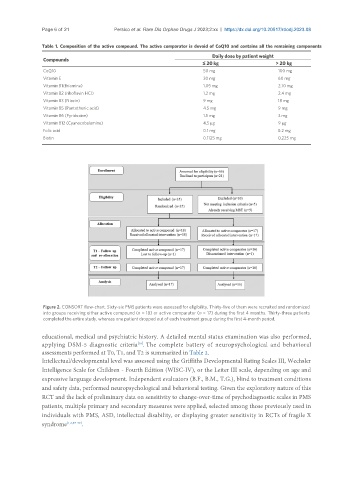
Figure 2. CONSORT flow-chart. Sixty-six PMS patients were assessed for eligibility. Thirty-five of them were recruited and randomized
into groups receiving either active compound (n = 18) or active comparator (n = 17) during the first 4 months. Thirty-three patients
completed the entire study, whereas one patient dropped out of each treatment group during the first 4-month period.
educational, medical and psychiatric history. A detailed mental status examination was also performed,
applying DSM-5 diagnostic criteria . The complete battery of neuropsychological and behavioral
[36]
assessments performed at T0, T1, and T2 is summarized in Table 2.
Intellectual/developmental level was assessed using the Griffiths Developmental Rating Scales III, Wechsler
Intelligence Scale for Children - Fourth Edition (WISC-IV), or the Leiter III scale, depending on age and
expressive language development. Independent evaluators (B.F., B.M., T.G.), blind to treatment conditions
and safety data, performed neuropsychological and behavioral testing. Given the exploratory nature of this
RCT and the lack of preliminary data on sensitivity to change-over-time of psychodiagnostic scales in PMS
patients, multiple primary and secondary measures were applied, selected among those previously used in
individuals with PMS, ASD, intellectual disability, or displaying greater sensitivity in RCTs of fragile X
syndrome [1,4,37-39] .