Page 49 - Read Online
P. 49
Persico et al. Rare Dis Orphan Drugs J 2023;2:xx https://dx.doi.org/10.20517/rdodj.2023.08 Page 5 of 21
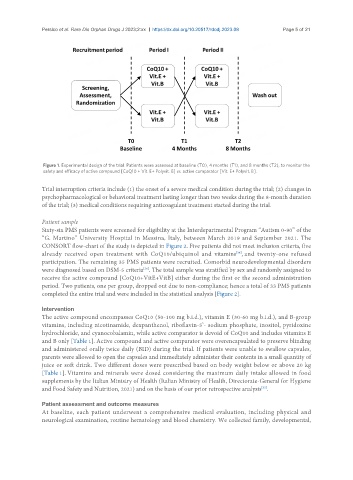
Figure 1. Experimental design of the trial. Patients were assessed at baseline (T0), 4 months (T1), and 8 months (T2), to monitor the
safety and efficacy of active compound [CoQ10 + Vit. E+ Polyvit. B] vs. active comparator [Vit. E+ Polyvit. B].
Trial interruption criteria include (1) the onset of a severe medical condition during the trial; (2) changes in
psychopharmacological or behavioral treatment lasting longer than two weeks during the 8-month duration
of the trial; (3) medical conditions requiring anticoagulant treatment started during the trial.
Patient sample
Sixty-six PMS patients were screened for eligibility at the Interdepartmental Program “Autism 0-90” of the
“G. Martino” University Hospital in Messina, Italy, between March 2019 and September 2021. The
CONSORT flow-chart of the study is depicted in Figure 2. Five patients did not meet inclusion criteria, five
[34]
already received open treatment with CoQ10/ubiquinol and vitamins , and twenty-one refused
participation. The remaining 35 PMS patients were recruited. Comorbid neurodevelopmental disorders
[36]
were diagnosed based on DSM-5 criteria . The total sample was stratified by sex and randomly assigned to
receive the active compound [CoQ10+VitE+VitB] either during the first or the second administration
period. Two patients, one per group, dropped out due to non-compliance; hence a total of 33 PMS patients
completed the entire trial and were included in the statistical analysis [Figure 2].
Intervention
The active compound encompasses CoQ10 (50-100 mg b.i.d.), vitamin E (30-60 mg b.i.d.), and B-group
vitamins, including nicotinamide, dexpanthenol, riboflavin-5’- sodium phosphate, inositol, pyridoxine
hydrochloride, and cyanocobalamin, while active comparator is devoid of CoQ10 and includes vitamins E
and B only [Table 1]. Active compound and active comparator were overencapsulated to preserve blinding
and administered orally twice daily (BID) during the trial. If patients were unable to swallow capsules,
parents were allowed to open the capsules and immediately administer their contents in a small quantity of
juice or soft drink. Two different doses were prescribed based on body weight below or above 20 kg
[Table 1]. Vitamins and minerals were dosed considering the maximum daily intake allowed in food
supplements by the Italian Ministry of Health (Italian Ministry of Health, Directorate-General for Hygiene
and Food Safety and Nutrition, 2021) and on the basis of our prior retrospective analysis .
[35]
Patient assessment and outcome measures
At baseline, each patient underwent a comprehensive medical evaluation, including physical and
neurological examination, routine hematology and blood chemistry. We collected family, developmental,