Page 58 - Read Online
P. 58
Page 22 of 29 Teng et al. Microstructures 2023;3:2023019 https://dx.doi.org/10.20517/microstructures.2023.07
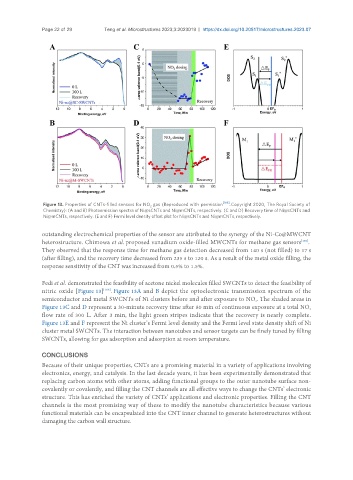
Figure 13. Properties of CNTs-filled sensors for NO gas (Reproduced with permission [166] . Copyright 2020, The Royal Society of
2
Chemistry): (A and B) Photoemission spectra of Ni@sCNTs and Ni@mCNTs, respectively. (C and D) Recovery time of Ni@sCNTs and
Ni@mCNTs, respectively. (E and F) Fermi level density offset plot for Ni@sCNTs and Ni@mCNTs, respectively.
outstanding electrochemical properties of the sensor are attributed to the synergy of the Ni-Co@MWCNT
[165]
heterostructure. Chimowa et al. proposed vanadium oxide-filled MWCNTs for methane gas sensors .
They observed that the response time for methane gas detection decreased from 140 s (not filled) to 17 s
(after filling), and the recovery time decreased from 235 s to 120 s. As a result of the metal oxide filling, the
response sensitivity of the CNT was increased from 0.5% to 1.5%.
Fedi et al. demonstrated the feasibility of acetone nickel molecules filled SWCNTs to detect the feasibility of
nitric oxide [Figure 13] . Figure 13A and B depict the optoelectronic transmission spectrum of the
[166]
semiconductor and metal SWCNTs of Ni clusters before and after exposure to NO . The shaded areas in
2
Figure 13C and D represent a 30-minute recovery time after 80 min of continuous exposure at a total NO
2
flow rate of 300 L. After 3 min, the light green stripes indicate that the recovery is nearly complete.
Figure 13E and F represent the Ni cluster’s Fermi level density and the Fermi level state density shift of Ni
cluster metal SWCNTs. The interaction between nanotubes and sensor targets can be finely tuned by filling
SWCNTs, allowing for gas adsorption and adsorption at room temperature.
CONCLUSIONS
Because of their unique properties, CNTs are a promising material in a variety of applications involving
electronics, energy, and catalysis. In the last decade years, it has been experimentally demonstrated that
replacing carbon atoms with other atoms, adding functional groups to the outer nanotube surface non-
covalently or covalently, and filling the CNT channels are all effective ways to change the CNTs’ electronic
structure. This has enriched the variety of CNTs’ applications and electronic properties. Filling the CNT
channels is the most promising way of these to modify the nanotube characteristics because various
functional materials can be encapsulated into the CNT inner channel to generate heterostructures without
damaging the carbon wall structure.