Page 14 - Read Online
P. 14
Page 10 of 21 Liu et al. Microstructures 2023;3:2023001 https://dx.doi.org/10.20517/microstructures.2022.23
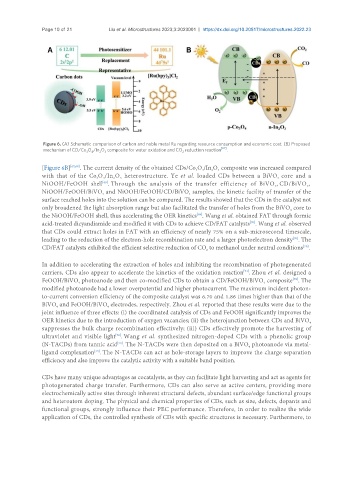
Figure 6. (A) Schematic comparison of carbon and noble metal Ru regarding resource consumption and economic cost. (B) Proposed
mechanism of CD/Co O /In O composite for water oxidation and CO reduction reaction [67] .
4
2
2
3
3
[Figure 6B] [67,68] . The current density of the obtained CDs/Co O /In O composite was increased compared
3
3
2
4
with that of the Co O /In O heterostructure. Ye et al. loaded CDs between a BiVO core and a
3
2
4
4
3
NiOOH/FeOOH shell . Through the analysis of the transfer efficiency of BiVO , CD/BiVO ,
[69]
4
4
NiOOH/FeOOH/BiVO and NiOOH/FeOOH/CD/BiVO samples, the kinetic facility of transfer of the
4
4
surface reached holes into the solution can be compared. The results showed that the CDs in the catalyst not
only broadened the light absorption range but also facilitated the transfer of holes from the BiVO core to
4
the NiOOH/FeOOH shell, thus accelerating the OER kinetics . Wang et al. obtained FAT through formic
[69]
[70]
acid-treated dicyandiamide and modified it with CDs to achieve CD/FAT catalysts . Wang et al. observed
that CDs could extract holes in FAT with an efficiency of nearly 75% on a sub-microsecond timescale,
leading to the reduction of the electron-hole recombination rate and a larger photoelectron density . The
[70]
CD/FAT catalysts exhibited the efficient selective reduction of CO to methanol under neutral conditions .
[70]
2
In addition to accelerating the extraction of holes and inhibiting the recombination of photogenerated
[71]
carriers, CDs also appear to accelerate the kinetics of the oxidation reaction . Zhou et al. designed a
FeOOH/BiVO photoanode and then co-modified CDs to obtain a CD/FeOOH/BiVO composite . The
[72]
4
4
modified photoanode had a lower overpotential and higher photocurrent. The maximum incident photon-
to-current conversion efficiency of the composite catalyst was 6.70 and 1.86 times higher than that of the
BiVO and FeOOH/BiVO electrodes, respectively. Zhou et al. reported that these results were due to the
4
4
joint influence of three effects: (i) the coordinated catalysis of CDs and FeOOH significantly improves the
OER kinetics due to the introduction of oxygen vacancies; (ii) the heterojunction between CDs and BiVO
4
suppresses the bulk charge recombination effectively; (iii) CDs effectively promote the harvesting of
[72]
ultraviolet and visible light . Wang et al. synthesized nitrogen-doped CDs with a phenolic group
(N-TACDs) from tannic acid . The N-TACDs were then deposited on a BiVO photoanode via metal-
[73]
4
ligand complexation . The N-TACDs can act as hole-storage layers to improve the charge separation
[73]
efficiency and also improve the catalytic activity with a suitable band position.
CDs have many unique advantages as cocatalysts, as they can facilitate light harvesting and act as agents for
photogenerated charge transfer. Furthermore, CDs can also serve as active centers, providing more
electrochemically active sites through inherent structural defects, abundant surface/edge functional groups
and heteroatom doping. The physical and chemical properties of CDs, such as size, defects, dopants and
functional groups, strongly influence their PEC performance. Therefore, in order to realize the wide
application of CDs, the controlled synthesis of CDs with specific structures is necessary. Furthermore, to