Page 382 - Read Online
P. 382
Page 2 of 6 Pal et al. Vessel Plus 2019;3:39 I http://dx.doi.org/10.20517/2574-1209.2019.24
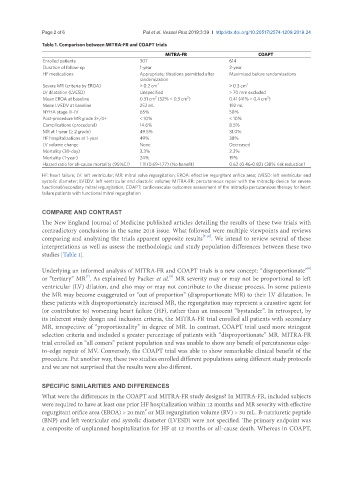
Table 1. Comparison between MITRA-FR and COAPT trials
MITRA-FR COAPT
Enrolled patients 307 614
Duration of follow-up 1-year 2-year
HF medications Appropriate; titrations permitted after Maximized before randomizations
randomization
Severe MR (criteria by EROA) > 0.2 cm 2 > 0.3 cm 2
LV dilatation (LVESD) Unspecified > 70 mm excluded
2
2
2
Mean EROA at baseline 0.31 cm (52% < 0.3 cm ) 0.41 (41% ≥ 0.4 cm )
Mean LVEDV at baseline 252 mL 192 mL
NYHA stage III-IV 65% 58%
Post-procedure MR grade 3+/4+ < 10% < 10%
Complications (procedural) 14.6% 8.5%
MR at 1-year (≥ 2 grade) 49.5% 31.0%
HF hospitalizations at 1-year 49% 38%
LV volume change None Decreased
Mortality (30-day) 3.3% 2.3%
Mortality (1-year) 24% 19%
Hazard ratio for all-cause mortality (95%CI) 1.11 (0.69-1.77) (No benefit) 0.62 (0.46-0.82) (38% risk reduction)
HF: heart failure; LV: left ventricular; MR: mitral valve regurgitation; EROA: effective regurgitant orifice area; LVESD: left ventricular end
systolic diameter; LVEDV: left ventricular end diastolic volume; MITRA-FR: percutaneous repair with the mitraclip device for severe
functional/secondary mitral regurgitation; COAPT: cardiovascular outcomes assessment of the mitraclip percutaneous therapy for heart
failure patients with functional mitral regurgitation
COMPARE AND CONTRAST
The New England Journal of Medicine published articles detailing the results of these two trials with
contradictory conclusions in the same 2018 issue. What followed were multiple viewpoints and reviews
comparing and analyzing the trials apparent opposite results [7-10] . We intend to review several of these
interpretations as well as assess the methodologic and study population differences between these two
studies [Table 1].
[8]
Underlying an informed analysis of MITRA-FR and COAPT trials is a new concept: “disproportionate”
or “tertiary” MR . As explained by Packer et al. MR severity may or may not be proportional to left
[7]
[8]
ventricular (LV) dilation, and also may or may not contribute to the disease process. In some patients
the MR may become exaggerated or “out of proportion” (disproportionate MR) to their LV dilatation. In
these patients with disproportionately increased MR, the regurgitation may represent a causative agent for
(or contributor to) worsening heart failure (HF), rather than an innocent “bystander”. In retrospect, by
its inherent study design and inclusion criteria, the MITRA-FR trial enrolled all patients with secondary
MR, irrespective of “proportionality” in degree of MR. In contrast, COAPT trial used more stringent
selection criteria and included a greater percentage of patients with “disproportionate” MR. MITRA-FR
trial enrolled an “all comers” patient population and was unable to show any benefit of percutaneous edge-
to-edge repair of MV. Conversely, the COAPT trial was able to show remarkable clinical benefit of the
procedure. Put another way, these two studies enrolled different populations using different study protocols
and we are not surprised that the results were also different.
SPECIFIC SIMILARITIES AND DIFFERENCES
What were the differences in the COAPT and MITRA-FR study designs? In MITRA-FR, included subjects
were required to have at least one prior HF hospitalization within 12 months and MR severity with effective
regurgitant orifice area (EROA) > 20 mm or MR regurgitation volume (RV) > 30 mL. B-natriuretic peptide
2
(BNP) and left ventricular end systolic diameter (LVESD) were not specified. The primary endpoint was
a composite of unplanned hospitalization for HF at 12 months or all-cause death. Whereas in COAPT,