Page 294 - Read Online
P. 294
Zimmermann et al. Vessel Plus 2019;3:31 I http://dx.doi.org/10.20517/2574-1209.2019.010 Page 7 of 18
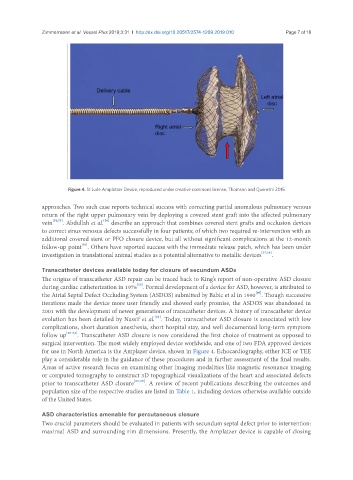
Figure 4. St Jude Amplatzer Device, reproduced under creative commons license, Thomson and Quereshi 2015
approaches. Two such case reports technical success with correcting partial anomalous pulmonary venous
return of the right upper pulmonary vein by deploying a covered stent graft into the affected pulmonary
[56]
vein [54,55] . Abdullah et al. describe an approach that combines covered stent grafts and occlusion devices
to correct sinus venosus defects successfully in four patients; of which two required re-intervention with an
additional covered stent or PFO closure device, but all without significant complications at the 12-month
[56]
follow-up point . Others have reported success with the immediate release patch, which has been under
investigation in translational animal studies as a potential alternative to metallic devices [57,58] .
Transcatheter devices available today for closure of secundum ASDs
The origins of transcatheter ASD repair can be traced back to King’s report of non-operative ASD closure
[59]
during cardiac catheterization in 1976 . Formal development of a device for ASD, however, is attributed to
[60]
the Atrial Septal Defect Occluding System (ASDOS) submitted by Babic et al in 1990 . Though successive
iterations made the device more user friendly and showed early promise, the ASDOS was abandoned in
2001 with the development of newer generations of transcatheter devices. A history of transcatheter device
evolution has been detailed by Nassif et al. . Today, transcatheter ASD closure is associated with low
[61]
complications, short duration anesthesia, short hospital stay, and well documented long-term symptom
follow up [62-64] . Transcatheter ASD closure is now considered the first choice of treatment as opposed to
surgical intervention. The most widely employed device worldwide, and one of two FDA approved devices
for use in North America is the Amplazer device, shown in Figure 4. Echocardiography, either ICE or TEE
play a considerable role in the guidance of these procedures and in further assessment of the final results.
Areas of active research focus on examining other imaging modalities like magnetic resonance imaging
or computed tomography to construct 3D topographical visualizations of the heart and associated defects
prior to transcatheter ASD closure [65-69] . A review of recent publications describing the outcomes and
population size of the respective studies are listed in Table 1, including devices otherwise available outside
of the United States.
ASD characteristics amenable for percutaneous closure
Two crucial parameters should be evaluated in patients with secundum septal defect prior to intervention:
maximal ASD and surrounding rim dimensions. Presently, the Amplatzer device is capable of closing