Page 221 - Read Online
P. 221
Jagpal et al. Vessel Plus 2018;2:24 I http://dx.doi.org/10.20517/2574-1209.2018.27 Page 5 of 14
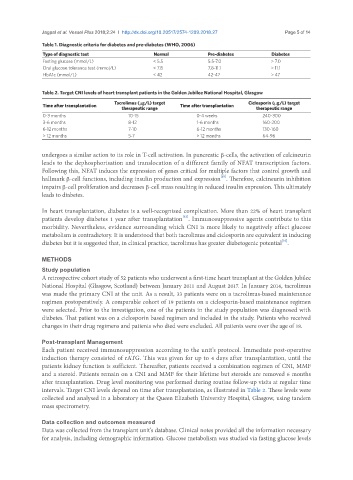
Table 1. Diagnostic criteria for diabetes and pre-diabetes (WHO, 2006)
Type of diagnostic test Normal Pre-diabetes Diabetes
Fasting glucose (mmol/L) < 5.5 5.5-7.0 > 7.0
Oral glucose tolerance test (mmol/L) < 7.8 7.8-11.1 > 11.1
HbA1c (mmol/L) < 42 42-47 > 47
Table 2. Target CNI levels of heart transplant patients in the Golden Jubilee National Hospital, Glasgow
Tacrolimus (mg/L) target Ciclosporin (mg/L) target
Time after transplantation Time after transplantation
therapeutic range therapeutic range
0-3 months 10-15 0-4 weeks 240-300
3-6 months 8-12 1-6 months 160-200
6-12 months 7-10 6-12 months 130-160
> 12 months 5-7 > 12 months 64-96
undergoes a similar action to its role in T-cell activation. In pancreatic b-cells, the activation of calcineurin
leads to the dephosphorisation and translocation of a different family of NFAT transcription factors.
Following this, NFAT induces the expression of genes critical for multiple factors that control growth and
hallmark b-cell functions, including insulin production and expression . Therefore, calcineurin inhibition
[19]
impairs b-cell proliferation and decreases b-cell mass resulting in reduced insulin expression. This ultimately
leads to diabetes.
In heart transplantation, diabetes is a well-recognised complication. More than 22% of heart transplant
[12]
patients develop diabetes 1 year after transplantation . Immunosuppressive agents contribute to this
morbidity. Nevertheless, evidence surrounding which CNI is more likely to negatively affect glucose
metabolism is contradictory. It is understood that both tacrolimus and ciclosporin are equivalent in inducing
diabetes but it is suggested that, in clinical practice, tacrolimus has greater diabetogenic potential .
[32]
METHODS
Study population
A retrospective cohort study of 52 patients who underwent a first-time heart transplant at the Golden Jubilee
National Hospital (Glasgow, Scotland) between January 2011 and August 2017. In January 2014, tacrolimus
was made the primary CNI at the unit. As a result, 33 patients were on a tacrolimus-based maintenance
regimen postoperatively. A comparable cohort of 19 patients on a ciclosporin-based maintenance regimen
were selected. Prior to the investigation, one of the patients in the study population was diagnosed with
diabetes. That patient was on a ciclosporin based regimen and included in the study. Patients who received
changes in their drug regimens and patients who died were excluded. All patients were over the age of 18.
Post-transplant Management
Each patient received immunosuppression according to the unit’s protocol. Immediate post-operative
induction therapy consisted of rATG. This was given for up to 4 days after transplantation, until the
patients kidney function is sufficient. Thereafter, patients received a combination regimen of CNI, MMF
and a steroid. Patients remain on a CNI and MMF for their lifetime but steroids are removed 6 months
after transplantation. Drug level monitoring was performed during routine follow-up visits at regular time
intervals. Target CNI levels depend on time after transplantation, as illustrated in Table 2. These levels were
collected and analysed in a laboratory at the Queen Elizabeth University Hospital, Glasgow, using tandem
mass spectrometry.
Data collection and outcomes measured
Data was collected from the transplant unit’s database. Clinical notes provided all the information necessary
for analysis, including demographic information. Glucose metabolism was studied via fasting glucose levels