Page 168 - Read Online
P. 168
Bradshaw et al. Vessel Plus 2023;7:35 https://dx.doi.org/10.20517/2574-1209.2023.121 Page 5 of 21
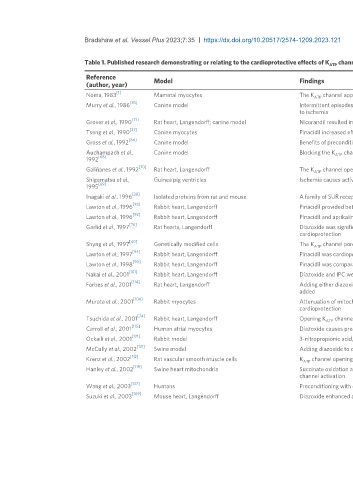
Table 1. Published research demonstrating or relating to the cardioprotective effects of K ATP channel openers
Reference Model Findings
(author, year)
[1]
Noma, 1983 Mammal myocytes The K channel appeared important for regulation of cellular energy metabolism in cardiac cells
ATP
[83]
Murry et al., 1986 Canine model Intermittent episodes of ischemia protected the myocardium by delaying cell death when the myocardium was later subjected
to ischemia
[71]
Grover et al., 1990 Rat heart, Langendorff; canine model Nicorandil resulted in improved contractility via indirect action, but cromakalim was directly cardioprotective
[37]
Tseng et al., 1990 Canine myocytes Pinacidil increased efflux through K channels, and this was modulated by enzymatic reaction
ATP
[84]
Gross et al., 1992 Canine model Benefits of preconditioning in dogs were abolished by blocking the K channel
ATP
Auchampach et al., Canine model Blocking the K ATP channel prevented benefits of IPC on infarct size after prolonged coronary occlusion
[68]
1992
[70] +
Galiñanes et al., 1992 Rat heart, Langendorff The K ATP channel opener lemakalim had anti-ischemic effects, but not in combination with high K cardioplegia
Shigematsu et al., Guinea pig ventricles Ischemia causes activation of K ATP channels, which contributes of recovery of contraction after reperfusion
[69]
1995
[38]
Inagaki et al., 1996 Isolated proteins from rat and mouse A family of SUR receptors determines the function of K channels
ATP
[93]
Lawton et al., 1996 Rabbit heart, Langendorff Pinacidil provided better postischemic recovery compared with controls
[92]
Lawton et al., 1996 Rabbit heart, Langendorff Pinacidil and aprikalim are comparable to St. Thomas’ solution for cardioprotection
[26]
Garlid et al., 1997 Rat hearts, Langendorff Diazoxide was significantly more potent than sarcolemmal K ATP at opening mitoK ATP , implicating a role for mitoK ATP in
cardioprotection
[40]
Shyng et al., 1997 Genetically modified cells The K channel pore is octameric or tetradimeric in structure. Each includes four Kir6.2 subunits and SUR1 subunits
ATP
[94]
Lawton et al., 1997 Rabbit heart, Langendorff Pinacidil was cardioprotective, and this cardioprotection was lost with a K blocker
ATP
[90]
Lawton et al., 1998 Rabbit heart, Langendorff Pinacidil was comparative to warm blood cardioplegia for systolic recovery
[101]
Nakai et al., 2001 Rabbit heart, Langendorff Diazoxide and IPC were both cardioprotective. Effects were lost with a mitoK channel blocker
ATP
[114]
Forbes et al., 2001 Rat heart, Langendorff Adding either diazoxide or pinacidil caused increased ROS, and this is blocked when 5-hydroxydecanoate or antioxidant is
added
[104] 2+
Murata et al., 2001 Rabbit myocytes Attenuation of mitochondrial Ca overload, because of partial mitochondrial depolarization by mitoK channels, provided
ATP
cardioprotection
[74]
Tsuchida et al., 2001 Rabbit heart, Langendorff Opening K ATP channels before ischemia and during early ischemia, but not upon reperfusion, was important for cardioprotection
[115]
Carroll et al., 2001 Human atrial myocytes Diazoxide causes preconditioning via mitochondrial swelling and free radical production
[121]
Ockaili et al., 2001 Rabbit model 3-nitropropionic acid, a mitochondrial SDH inhibitor, has anti-ischemic effects due to mitoK ATP channel opening
[131]
McCully et al., 2002 Swine model Adding diazoxide to cardioplegia decreased myocardial apoptosis and mitochondrial damage
[112]
Krenz et al., 2002 Rat vascular smooth muscle cells K ATP channel opening by diazoxide or pinacidil led to ROS production from mitochondria
[119]
Hanley et al., 2002 Swine heart mitochondria Succinate oxidation and SDH activity were inhibited by diazoxide, suggesting a cardioprotective mechanism other than K ATP
channel activation
[137]
Wang et al., 2003 Humans Preconditioning with diazoxide was protective compared to cardioplegia alone, resulting in better hemodynamic recovery
[109]
Suzuki et al., 2003 Mouse heart, Langendorff Diazoxide enhanced actional potential shortening during ischemia by activating sarcolemmal K channels
ATP