Page 54 - Read Online
P. 54
Jung et al. Soft Sci 2024;4:15 https://dx.doi.org/10.20517/ss.2024.02 Page 33 of 44
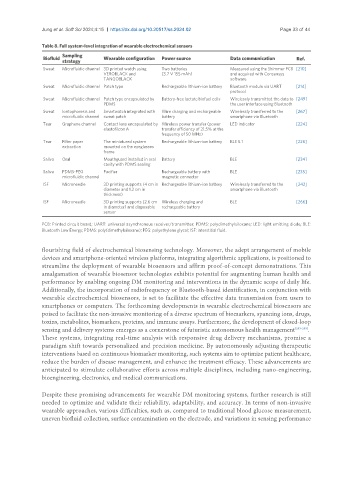
Table 8. Full system-level integration of wearable electrochemical sensors
Sampling
Biofluid Wearable configuration Power source Data communication Ref.
strategy
Sweat Microfluidic channel 3D printed watch using Two batteries Measured using the Shimmer PCB [210]
VEROBLACK and (3.7 V 155 mAh) and acquired with Consensys
TANGOBLACK software
Sweat Microfluidic channel Patch type Rechargeable lithium-ion battery Bluetooth module via UART [214]
protocol
Sweat Microfluidic channel Patch type encapsulated by Battery-free lactate biofuel cells Wirelessly transmitted the data to [249]
PDMS the user interface using Bluetooth
Sweat Iontophoresis and Smartwatch integrated with Wire charging and rechargeable Wirelessly transferred to the [267]
microfluidic channel sweat patch battery smartphone via Bluetooth
Tear Graphene channel Contact lens encapsulated by Wireless power transfer (power LED indicator [224]
elastofilcon A transfer efficiency of 21.5% at the
frequency of 50 MHz)
Tear Filter paper The miniatured system Rechargeable lithium-ion battery BLE 5.1 [226]
extraction mounted on the eyeglasses
frame
Saliva Oral Mouthguard installed in oral Battery BLE [234]
cavity with PDMS sealing
Saliva PDMS-PEG Pacifier Rechargeable battery with BLE [235]
microfluidic channel magnetic connector
ISF Microneedle 3D printing supports (4 cm in Rechargeable lithium-ion battery Wirelessly transferred to the [242]
diameter and 1.2 cm in smartphone via Bluetooth
thickness)
ISF Microneedle 3D printing supports (2.6 cm Wireless charging and BLE [266]
in diameter) and disposable rechargeable battery
sensor
PCB: Printed circuit board; UART: universal asynchronous receiver/transmitter; PDMS: polydimethylsiloxane; LED: light emitting diode; BLE:
Bluetooth Low Energy; PDMS: poly(dimethylsiloxane); PEG: polyethylene glycol; ISF: interstitial fluid .
flourishing field of electrochemical biosensing technology. Moreover, the adept arrangement of mobile
devices and smartphone-oriented wireless platforms, integrating algorithmic applications, is positioned to
streamline the deployment of wearable biosensors and affirm proof-of-concept demonstrations. This
amalgamation of wearable biosensor technologies exhibits potential for augmenting human health and
performance by enabling ongoing DM monitoring and interventions in the dynamic scope of daily life.
Additionally, the incorporation of radiofrequency or Bluetooth-based identification, in conjunction with
wearable electrochemical biosensors, is set to facilitate the effective data transmission from users to
smartphones or computers. The forthcoming developments in wearable electrochemical biosensors are
poised to facilitate the non-invasive monitoring of a diverse spectrum of biomarkers, spanning ions, drugs,
toxins, metabolites, biomarkers, proteins, and immune assays. Furthermore, the development of closed-loop
sensing and delivery systems emerges as a cornerstone of futuristic autonomous health management [283-285] .
These systems, integrating real-time analysis with responsive drug delivery mechanisms, promise a
paradigm shift towards personalized and precision medicine. By autonomously adjusting therapeutic
interventions based on continuous biomarker monitoring, such systems aim to optimize patient healthcare,
reduce the burden of disease management, and enhance the treatment efficacy. These advancements are
anticipated to stimulate collaborative efforts across multiple disciplines, including nano-engineering,
bioengineering, electronics, and medical communications.
Despite these promising advancements for wearable DM monitoring systems, further research is still
needed to optimize and validate their reliability, adaptability, and accuracy. In terms of non-invasive
wearable approaches, various difficulties, such as, compared to traditional blood glucose measurement,
uneven biofluid collection, surface contamination on the electrode, and variations in sensing performance