Page 22 - Read Online
P. 22
Kim et al. Plast Aesthet Res 2018;5:31 I http://dx.doi.org/10.20517/2347-9264.2018.26 Page 3 of 8
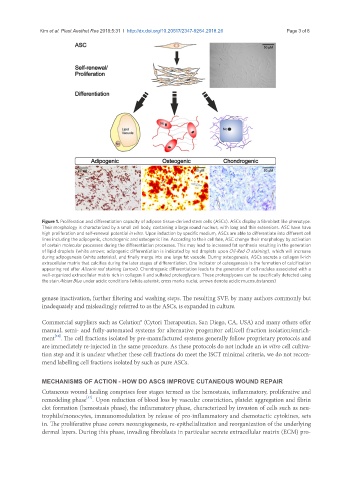
Figure 1. Proliferation and differentiation capacity of adipose tissue-derived stem cells (ASCs). ASCs display a fibroblast like phenotype.
Their morphology is characterized by a small cell body, containing a large round nucleus, with long and thin extensions. ASC have have
high proliferation and self-renewal potential in vitro. Upon induction by specific medium, ASCs are able to differentiate into different cell
lines including the adipogenic, chondrogenic and osteogenic line. According to their cell fate, ASC change their morphology by activation
of certain molecular processes during the differentiation processes. This may lead to increased fat synthesis resulting in the generation
of lipid droplets (white arrows; adipogenic differentiation is indicated by red droplets upon Oil-Red O staining), which will increase
during adipogenesis (white asterisks), and finally merge into one large fat vacuole. During osteogenesis, ASCs secrete a collagen I-rich
extracellular matrix that calcifies during the later stages of differentiation. One indicator of osteogenesis is the formation of calcification
appearing red after Alizarin red staining (arrow). Chondrogenic differentiation leads to the generation of cell nodules associated with a
well-organized extracellular matrix rich in collagen II and sulfated proteoglycans. These proteoglycans can be specifically detected using
the stain Alcian Blue under acidic conditions (white asterisk; cross marks nuclei; arrows denote acidic mucosubstances)
genase inactivation, further filtering and washing steps. The resulting SVF, by many authors commonly but
inadequately and misleadingly referred to as the ASCs, is expanded in culture.
Commercial suppliers such as Celution® (Cytori Therapeutics, San Diego, CA, USA) and many others offer
manual, semi- and fully-automated systems for alternative progenitor cell/cell fraction isolation/enrich-
[16]
ment . The cell fractions isolated by pre-manufactured systems generally follow proprietary protocols and
are immediately re-injected in the same procedure. As these protocols do not include an in vitro cell cultiva-
tion step and it is unclear whether these cell fractions do meet the ISCT minimal criteria, we do not recom-
mend labelling cell fractions isolated by such as pure ASCs.
MECHANISMS OF ACTION - HOW DO ASCS IMPROVE CUTANEOUS WOUND REPAIR
Cutaneous wound healing comprises four stages termed as the hemostasis, inflammatory, proliferative and
[17]
remodeling phase . Upon reduction of blood loss by vascular constriction, platelet aggregation and fibrin
clot formation (hemostasis phase), the inflammatory phase, characterized by invasion of cells such as neu-
trophils/monocytes, immunomodulation by release of pro-inflammatory and chemotactic cytokines, sets
in. The proliferative phase covers neoangiogenesis, re-epithelialization and reorganization of the underlying
dermal layers. During this phase, invading fibroblasts in particular secrete extracellular matrix (ECM) pro-