Page 103 - Read Online
P. 103
Jaswant et al. One Health Implement Res 2024;4:15-37 https://dx.doi.org/10.20517/ohir.2023.61 Page 23
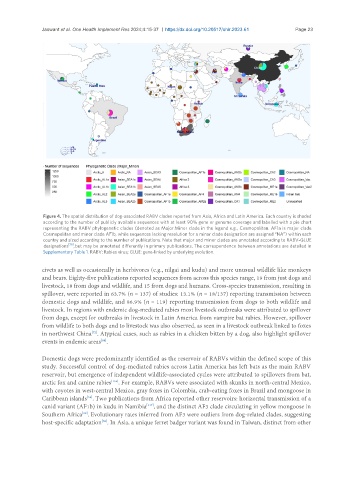
Figure 4. The spatial distribution of dog-associated RABV clades reported from Asia, Africa and Latin America. Each country is shaded
according to the number of publicly available sequences with at least 90% gene or genome coverage and labelled with a pie chart
representing the RABV phylogenetic clades (denoted as Major.Minor clade in the legend e.g., Cosmopolitan. AF1a is major clade
Cosmopolitan and minor clade AF1b, while sequences lacking resolution for a minor clade designation are assigned “NA”) within each
country and sized according to the number of publications. Note that major and minor clades are annotated according to RABV-GLUE
designations [15] , but may be annotated differently in primary publications. The correspondence between annotations are detailed in
Supplementary Table 1. RABV: Rabies virus; GLUE: gene-linked by underlying evolution.
civets as well as occasionally in herbivores (e.g., nilgai and kudu) and more unusual wildlife like monkeys
and bears. Eighty-five publications reported sequences from across this species range, 19 from just dogs and
livestock, 18 from dogs and wildlife, and 15 from dogs and humans. Cross-species transmission, resulting in
spillover, were reported in 63.7% (n = 137) of studies: 13.1% (n = 18/137) reporting transmission between
domestic dogs and wildlife, and 86.9% (n = 119) reporting transmission from dogs to both wildlife and
livestock. In regions with endemic dog-mediated rabies most livestock outbreaks were attributed to spillover
from dogs, except for outbreaks in livestock in Latin America from vampire bat rabies. However, spillover
from wildlife to both dogs and to livestock was also observed, as seen in a livestock outbreak linked to foxes
[72]
in northwest China . Atypical cases, such as rabies in a chicken bitten by a dog, also highlight spillover
events in endemic areas .
[84]
Domestic dogs were predominantly identified as the reservoir of RABVs within the defined scope of this
study. Successful control of dog-mediated rabies across Latin America has left bats as the main RABV
reservoir, but emergence of independent wildlife-associated cycles were attributed to spillovers from bat,
arctic fox and canine rabies . For example, RABVs were associated with skunks in north-central Mexico,
[116]
with coyotes in west-central Mexico, gray foxes in Colombia, crab-eating foxes in Brazil and mongoose in
Caribbean islands . Two publications from Africa reported other reservoirs: horizontal transmission of a
[56]
canid variant (AF1b) in kudu in Namibia , and the distinct AF3 clade circulating in yellow mongoose in
[117]
[62]
Southern Africa . Evolutionary rates inferred from AF3 were outliers from dog-related clades, suggesting
host-specific adaptation . In Asia, a unique ferret badger variant was found in Taiwan, distinct from other
[52]