Page 20 - Read Online
P. 20
Ong et al. Neuroimmunol Neuroinflammation 2020;7:311-8 I http://dx.doi.org/10.20517/2347-8659.2020.05 Page 315
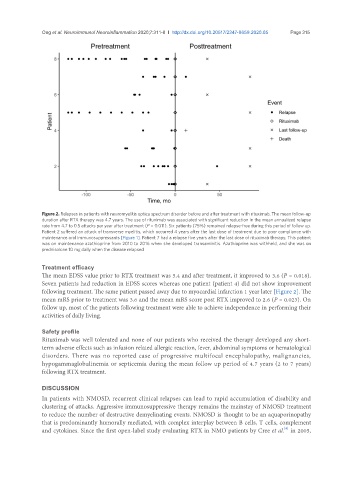
Figure 2. Relapses in patients with neuromyelitis optica spectrum disorder before and after treatment with rituximab. The mean follow-up
duration after RTX therapy was 4.7 years. The use of rituximab was associated with significant reduction in the mean annualized relapse
rate from 4.7 to 0.5 attacks per year after treatment (P = 0.011). Six patients (75%) remained relapse-free during this period of follow up.
Patient 2 suffered an attack of transverse myelitis, which occurred 4 years after the last dose of treatment due to poor compliance with
maintenance oral immunosuppressants [Figure 1]. Patient 7 had a relapse five years after the last dose of rituximab therapy. This patient
was on maintenance azathioprine from 2010 to 2016 when she developed transaminitis. Azathioprine was withheld, and she was on
prednisolone 10 mg daily when the disease relapsed
Treatment efficacy
The mean EDSS value prior to RTX treatment was 5.4 and after treatment, it improved to 3.6 (P = 0.018).
Seven patients had reduction in EDSS scores whereas one patient (patient 4) did not show improvement
following treatment. The same patient passed away due to myocardial infarction 1 year later [Figure 2]. The
mean mRS prior to treatment was 3.6 and the mean mRS score post RTX improved to 2.6 (P = 0.023). On
follow up, most of the patients following treatment were able to achieve independence in performing their
activities of daily living.
Safety profile
Rituximab was well tolerated and none of our patients who received the therapy developed any short-
term adverse effects such as infusion related allergic reaction, fever, abdominal symptoms or hematological
disorders. There was no reported case of progressive multifocal encephalopathy, malignancies,
hypogammaglobulinemia or septicemia during the mean follow up period of 4.7 years (2 to 7 years)
following RTX treatment.
DISCUSSION
In patients with NMOSD, recurrent clinical relapses can lead to rapid accumulation of disability and
clustering of attacks. Aggressive immunosuppressive therapy remains the mainstay of NMOSD treatment
to reduce the number of destructive demyelinating events. NMOSD is thought to be an aquaporinopathy
that is predominantly humorally mediated, with complex interplay between B cells, T cells, complement
[6]
and cytokines. Since the first open-label study evaluating RTX in NMO patients by Cree et al. in 2005,