Page 12 - Read Online
P. 12
Davis et al. Neuroimmunol Neuroinflammation 2020;7:300-10 I http://dx.doi.org/10.20517/2347-8659.2020.19 Page 307
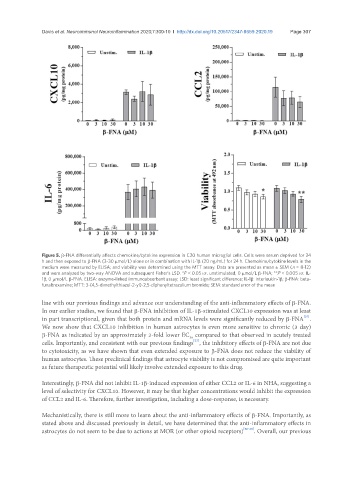
Figure 5. β-FNA differentially affects chemokine/cytokine expression in C20 human microglial cells. Cells were serum deprived for 24
h and then exposed to β-FNA (3-30 µmol/L) alone or in combination with IL-1β (20 ng/mL) for 24 h. Chemokine/cytokine levels in the
medium were measured by ELISA; and viability was determined using the MTT assay. Data are presented as mean ± SEM (n = 8-12)
and were analyzed by two-way ANOVA and subsequent Fisher’s LSD. *P < 0.05 vs. unstimulated, 0 µmol/L β-FNA; **P < 0.005 vs. IL-
1β, 0 µmol/L β-FNA. ELISA: enzyme-linked immunoabsorbant assay; LSD: least significant difference; IL-1β: interleukin-1β; β-FNA: beta-
funaltrexamine; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SEM: standard error of the mean
line with our previous findings and advance our understanding of the anti-inflammatory effects of β-FNA.
In our earlier studies, we found that β-FNA inhibition of IL-1β-stimulated CXCL10 expression was at least
[37]
in part transcriptional, given that both protein and mRNA levels were significantly reduced by β-FNA .
We now show that CXCL10 inhibition in human astrocytes is even more sensitive to chronic (3 day)
β-FNA as indicated by an approximately 3-fold lower EC compared to that observed in acutely treated
50
[27]
cells. Importantly, and consistent with our previous findings , the inhibitory effects of β-FNA are not due
to cytotoxicity, as we have shown that even extended exposure to β-FNA does not reduce the viability of
human astrocytes. These preclinical findings that astrocyte viability is not compromised are quite important
as future therapeutic potential will likely involve extended exposure to this drug.
Interestingly, β-FNA did not inhibit IL-1β-induced expression of either CCL2 or IL-6 in NHA, suggesting a
level of selectivity for CXCL10. However, it may be that higher concentrations would inhibit the expression
of CCL2 and IL-6. Therefore, further investigation, including a dose-response, is necessary.
Mechanistically, there is still more to learn about the anti-inflammatory effects of β-FNA. Importantly, as
stated above and discussed previously in detail, we have determined that the anti-inflammatory effects in
astrocytes do not seem to be due to actions at MOR (or other opioid receptors) [26-28] . Overall, our previous