Page 63 - Read Online
P. 63
Victor et al. Neuroimmunol Neuroinflammation 2020;7:234-47 I http://dx.doi.org/10.20517/2347-8659.2020.02 Page 241
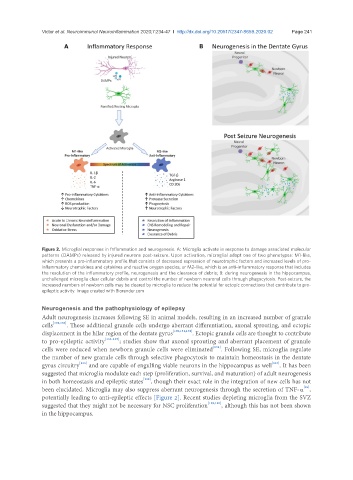
Figure 2. Microglial responses in fnflammation and neurogenesis. A: Microglia activate in response to damage associated molecular
patterns (DAMPs) released by injured neurons post-seizure. Upon activation, microglial adopt one of two phenotypes: M1-like,
which presents a pro-inflammatory profile that consists of decreased expression of neurotrophic factors and increased levels of pro-
inflammatory chemokines and cytokines and reactive oxygen species, or M2-like, which is an anti-inflammatory response that includes
the resolution of the inflammatory profile, neurogenesis and the clearance of debris; B: during neurogenesis in the hippocampus,
unchallenged microglia clear cellular debris and control the number of newborn neuronal cells through phagocytosis. Post-seizure, the
increased numbers of newborn cells may be cleared by microglia to reduce the potential for ectopic connections that contribute to pro-
epileptic activity. Image created with Biorender.com
Neurogenesis and the pathophysiology of epilepsy
Adult neurogenesis increases following SE in animal models, resulting in an increased number of granule
cells [129,130] . These additional granule cells undergo aberrant differentiation, axonal sprouting, and ectopic
displacement in the hilar region of the dentate gyrus [109,131,132] . Ectopic granule cells are thought to contribute
to pro-epileptic activity [133-135] ; studies show that axonal sprouting and aberrant placement of granule
cells were reduced when newborn granule cells were eliminated [132] . Following SE, microglia regulate
the number of new granule cells through selective phagocytosis to maintain homeostasis in the dentate
gyrus circuitry [136] and are capable of engulfing viable neurons in the hippocampus as well [137] . It has been
suggested that microglia modulate each step (proliferation, survival, and maturation) of adult neurogenesis
in both homeostasis and epileptic states [138] , though their exact role in the integration of new cells has not
[95]
been elucidated. Microglia may also suppress aberrant neurogenesis through the secretion of TNF-α ,
potentially leading to anti-epileptic effects [Figure 2]. Recent studies depleting microglia from the SVZ
suggested that they might not be necessary for NSC proliferation [139,140] , although this has not been shown
in the hippocampus.