Page 16 - Read Online
P. 16
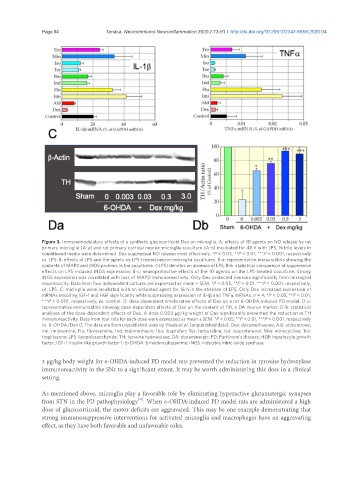
Page 84 Tanaka. Neuroimmunol Neuroinflammation 2020;7:73-91 I http://dx.doi.org/10.20517/2347-8659.2020.04
Figure 3. Immunomodulatory effects of a synthetic glucocorticoid Dex on microglia. A: effects of 10 agents on NO release by rat
primary microglia (A-a) and rat primary cortical neuron-microglia coculture (A-b) incubated for 48 h with LPS. Nitrite levels in
conditioned media were determined. Dex suppressed NO release most effectively. *P < 0.05, **P < 0.01, ***P < 0.001, respectively
vs . LPS. B: effects of LPS and the agents on LPS-treated neuron-microglia cocultures. B-a: representative immunoblots showing the
contents of MAP2 and iNOS proteins in the cocultures. (-LPS) denotes an absence of LPS; B-b: statistical comparison of suppressive
effects on LPS-induced iNOS expression; B-c: neuroprotective effects of the 10 agents on the LPS-treated coculture. Strong
iNOS expression was correlated with loss of MAP2-immunoreactivity. Only Dex protected neurons significantly from microglial
neurotoxicity. Data from four independent cultures are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, respectively,
vs . LPS. C: microglia were incubated with an indicated agent for 16 h in the absence of LPS. Only Dex increased expression of
mRNAs encoding IGF-1 and HGF significantly while suppressing expression of IL-1β and TNFa mRNAs. n = 4, *P < 0.05, **P < 0.01,
***P < 0.001, respectively, vs . control. D: dose-dependent ameliorative effects of Dex on a rat 6-OHDA-induced PD model. D-a:
representative immunoblots showing dose-dependent effects of Dex on the content of TH, a DA neuron marker; D-b: statistical
analyses of the dose-dependent effects of Dex. A dose 0.003 µg/kg weight of Dex significantly prevented the reduction in TH
immunoreactivity. Data from four rats for each dose were expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, respectively
vs . 6-OHDA/Dex 0. The data are from unpublished ones by Wada et al. (unpublished data). Dex: dexamethasone; Ald: aldosterone;
Imi: imipramine; Flu: fluvoxamine; Ind: indomethacin; Ibu: ibuprofen; Ter: terbutaline; Iso: isoproterenol; Min: minocycline; Tro:
troglitazone; LPS: lipopolysaccharide; TH: tyrosine hydroxylase; DA: dopaminergic; PD: Parkinson’s disease; HGF: hepatocyte growth
factor; IGF-1: insulin-like growth factor 1; 6-OHDA: 6-hydorxydopamine; iNOS: inducible nitric oxide synthase
3 µg/kg body weight for 6-OHDA-induced PD model rats prevented the reduction in tyrosine hydroxylase
immunoreactivity in the SNc to a significant extent. It may be worth administering this dose in a clinical
setting.
As mentioned above, microglia play a favorable role by eliminating hyperactive glutamatergic synapses
[47]
from STN in the PD pathophysiology . When 6-OHDA-induced PD model rats are administered a high
dose of glucocorticoid, the motor deficits are aggravated. This may be one example demonstrating that
strong immunosuppressive interventions for activated microglia and macrophages have an aggravating
effect, as they have both favorable and unfavorable roles.