Page 14 - Read Online
P. 14
Page 82 Tanaka. Neuroimmunol Neuroinflammation 2020;7:73-91 I http://dx.doi.org/10.20517/2347-8659.2020.04
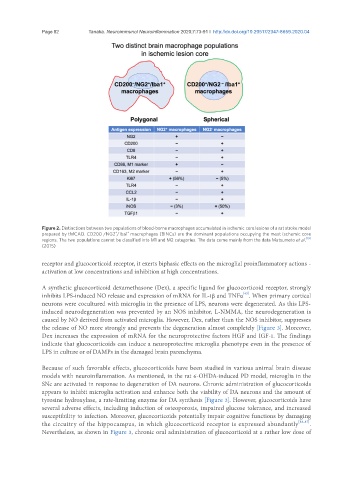
Figure 2. Distinctions between two populations of blood-borne macrophages accumulated in ischemic core lesions of a rat stroke model
+
+
prepared by tMCAO. CD200 /NG2 /Iba1 macrophages (BINCs) are the dominant populations occupying the most ischemic core
-
regions. The two populations cannot be classified into M1 and M2 categories. The data come mainly from the data Matsumoto et al. [19]
(2015)
receptor and glucocorticoid receptor, it exerts biphasic effects on the microglial proinflammatory actions -
activation at low concentrations and inhibition at high concentrations.
A synthetic glucocorticoid dexamethasone (Dex), a specific ligand for glucocorticoid receptor, strongly
[43]
inhibits LPS-induced NO release and expression of mRNA for IL-1β and TNFa . When primary cortical
neurons were cocultured with microglia in the presence of LPS, neurons were degenerated. As this LPS-
induced neurodegeneration was prevented by an NOS inhibitor, L-NMMA, the neurodegeneration is
caused by NO derived from activated microglia. However, Dex, rather than the NOS inhibitor, suppresses
the release of NO more strongly and prevents the degeneration almost completely [Figure 3]. Moreover,
Dex increases the expression of mRNA for the neuroprotective factors HGF and IGF-1. The findings
indicate that glucocorticoids can induce a neuroprotective microglia phenotype even in the presence of
LPS in culture or of DAMPs in the damaged brain parenchyma.
Because of such favorable effects, glucocorticoids have been studied in various animal brain disease
models with neuroinflammation. As mentioned, in the rat 6-OHDA-induced PD model, microglia in the
SNc are activated in response to degeneration of DA neurons. Chronic administration of glucocorticoids
appears to inhibit microglia activation and enhance both the viability of DA neurons and the amount of
tyrosine hydroxylase, a rate-limiting enzyme for DA synthesis [Figure 3]. However, glucocorticoids have
several adverse effects, including induction of osteoporosis, impaired glucose tolerance, and increased
susceptibility to infection. Moreover, glucocorticoids potentially impair cognitive functions by damaging
the circuitry of the hippocampus, in which glucocorticoid receptor is expressed abundantly [86,87] .
Nevertheless, as shown in Figure 3, chronic oral administration of glucocorticoid at a rather low dose of