Page 49 - Read Online
P. 49
Montabone et al. Neuroimmunol Neuroinflammation 2019;6:7 I http://dx.doi.org/10.20517/2347-8659.2019.09 Page 5 of 7
A B
C
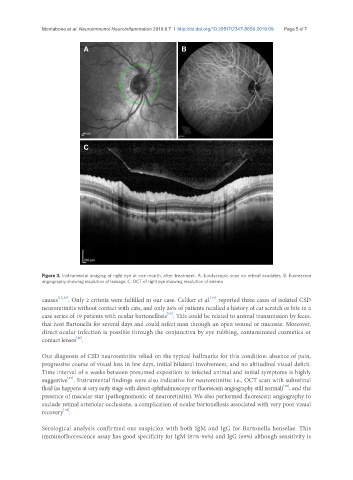
Figure 3. Instrumental imaging of right eye at one month, after treatment. A: funduscopic scan no retinal exudates; B: fluorescein
angiography showing resolution of leakage; C: OCT of right eye showing resolution of edema
[14]
causes [12,13] . Only 2 criteria were fulfilled in our case. Celiker et al. reported three cases of isolated CSD
neuroretinitis without contact with cats, and only 26% of patients recalled a history of cat scratch or bite in a
[15]
case series of 19 patients with ocular bartonellosis . This could be related to animal transmission by feces,
that host Bartonella for several days and could infect man through an open wound or mucosae. Moreover,
direct ocular infection is possible through the conjunctiva by eye rubbing, contaminated cosmetics or
[16]
contact lenses .
Our diagnosis of CSD neuroretinitis relied on the typical hallmarks for this condition: absence of pain,
progressive course of visual loss in few days, initial bilateral involvement, and no altitudinal visual deficit.
Time interval of 6 weeks between presumed exposition to infected animal and initial symptoms is highly
[17]
suggestive . Instrumental findings were also indicative for neuroretinitis: i.e., OCT scan with subretinal
[18]
fluid (as happens at very early stage with direct ophthalmoscopy or fluorescein angiography still normal) , and the
presence of macular star (pathognomonic of neuroretinitis). We also performed fluorescein angiography to
exclude retinal arteriolar occlusions, a complication of ocular bartonellosis associated with very poor visual
[19]
recovery .
Serological analysis confirmed our suspicion with both IgM and IgG for Bartonella henselae. This
immunofluorescence assay has good specificity for IgM (87%-96%) and IgG (69%) although sensitivity is