Page 150 - Read Online
P. 150
Zoghi et al. Neuroimmunol Neuroinflammation 2019;6:14 I http://dx.doi.org/10.20517/2347-8659.2019.03 Page 3 of 14
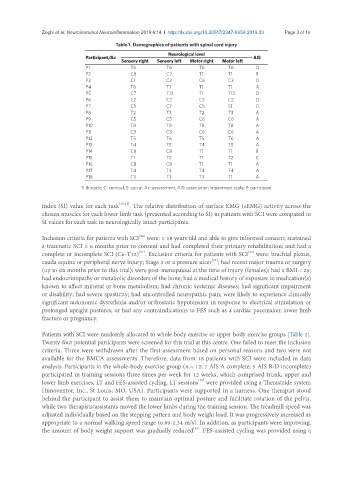
Table 1. Demographics of patients with spinal cord injury
Neurological level
Participant/Ax AIS
Sensory right Sensory left Motor right Motor left
P1 T6 T6 T6 T6 D
P2 C8 C7 T1 T1 B
P3 C1 C3 C6 C3 D
P4 T6 T7 T1 T1 A
P5 C7 T12 T1 T12 D
P6 C2 C2 C2 C2 D
P7 C5 C7 C5 S1 D
P8 T2 T3 T2 T3 A
P9 C5 C5 C6 C6 A
P10 T8 T8 T8 T8 A
P11 C3 C3 C6 C6 A
P12 T5 T6 T5 T6 A
P13 T4 T5 T4 T5 A
P14 C8 C8 T1 T1 B
P15 T1 T2 T1 T2 C
P16 C8 C8 T1 T1 A
P17 T4 T4 T4 T4 A
P18 T3 T3 T3 T1 A
T: thoracic; C: cervical; S: sacral; Ax: assessment; AIS: association impairment scale; P: participant
index (SI) value for each task [20,21] . The relative distribution of surface EMG (sEMG) activity across the
chosen muscles for each lower limb task (presented according to SI) in patients with SCI were compared to
SI values for each task in neurologically intact participants.
[22]
Inclusion criteria for patients with SCI were: ≥ 18 years old and able to give informed consent; sustained
a traumatic SCI ≥ 6 months prior to consent and had completed their primary rehabilitation; and had a
[22]
[17]
complete or incomplete SCI (C6-T12) . Exclusion criteria for patients with SCI were: brachial plexus,
[23]
cauda equina or peripheral nerve injury; Stage 3 or 4 pressure ulcer ; had recent major trauma or surgery
(up to six months prior to this trial); were post-menopausal at the time of injury (females); had a BMI < 25;
had endocrinopathy or metabolic disorders of the bone; had a medical history of exposure to medication(s)
known to affect mineral or bone metabolism; had chronic systemic diseases; had significant impairment
or disability; had severe spasticity; had uncontrolled neuropathic pain; were likely to experience clinically
significant autonomic dysreflexia and/or orthostatic hypotension in response to electrical stimulation or
prolonged upright postures; or had any contraindications to FES such as a cardiac pacemaker, lower limb
fracture or pregnancy.
Patients with SCI were randomly allocated to whole body exercise or upper body exercise groups [Table 2].
Twenty-four potential participants were screened for this trial at this centre. One failed to meet the inclusion
criteria. Three were withdrawn after the first assessment based on personal reasons and two were not
available for the BMCA assessments. Therefore, data from 18 patients with SCI were included in data
analysis. Participants in the whole-body exercise group (n = 12; 7 AIS A complete; 5 AIS B-D incomplete)
participated in training sessions three times per week for 12 weeks, which comprised trunk, upper and
[22]
lower limb exercises, LT and FES-assisted cycling. LT sessions were provided using a Therastride system
(Innoventor, Inc., St Louis, MO, USA). Participants were supported in a harness. One therapist stood
behind the participant to assist them to maintain optimal posture and facilitate rotation of the pelvis,
while two therapists/assistants moved the lower limbs during the training session. The treadmill speed was
adjusted individually based on the stepping pattern and body weight load. It was progressively increased as
appropriate to a normal walking speed range (0.89-1.34 m/s). In addition, as participants were improving,
[24]
the amount of body weight support was gradually reduced . FES-assisted cycling was provided using a