Page 397 - Read Online
P. 397
Pearce et al. Neuroimmunol Neuroinflammation 2018;5:47 I http://dx.doi.org/10.20517/2347-8659.2018.46 Page 9 of 16
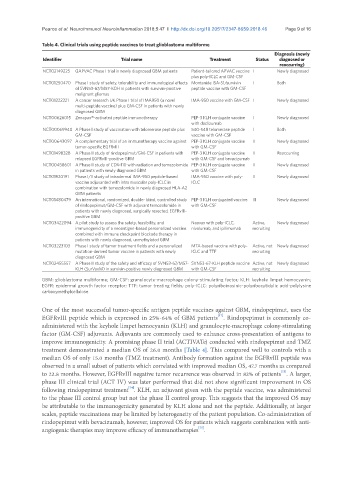
Table 4. Clinical trials using peptide vaccines to treat glioblastoma multiforme
Diagnosis (newly
Identifier Trial name Treatment Status diagnosed or
reoccurring)
NCT02149225 GAPVAC Phase I trial in newly diagnosed GBM patients Patient-tailored APVAC vaccine I Newly diagnosed
plus poly-ICLC and GM-CSF
NCT01250470 Phase I study of safety, tolerability and immunological effects Montanide ISA-51/survivin I Both
of SVN53-67/M57-KOH in patients with survivin-positive peptide vaccine with GM-CSF
malignant gliomas
NCT01222221 A cancer research UK Phase I trial of IMA950 (a novel IMA-950 vaccine with GM-CSF I Newly diagnosed
multi-peptide vaccine) plus GM-CSF in patients with newly
diagnosed GBM
NCT00626015 Zenapax®-activated peptide immunotherapy PEP-3 KLH conjugate vaccine I Newly diagnosed
with daclizumab
NCT00069940 A Phase I study of vaccination with telomerase peptide plus 540-548 telomerase peptide I Both
GM-CSF vaccine with GM-CSF
NCT00643097 A complementary trial of an immunotherapy vaccine against PEP-3 KLH conjugate vaccine II Newly diagnosed
tumor-specific EGFRvIII with GM-CSF
NCT01498328 A Phase II study of rindopepimut/GM-CSF in patients with PEP-3 KLH conjugate vaccine II Reoccurring
relapsed EGFRvIII-positive GBM with GM-CSF and bevacizumab
NCT00458601 A Phase II study of CDX-110 with radiation and temozolomide PEP-3 KLH conjugate vaccine II Newly diagnosed
in patients with newly diagnosed GBM with GM-CSF
NCT01920191 Phase I/II study of intradermal IMA-950 peptide-based IMA-950 vaccine with poly- II Newly diagnosed
vaccine adjuvanted with intra muscular poly-ICLC in ICLC
combination with temozolomide in newly diagnosed HLA-A2
GBM patients
NCT01480479 An international, randomized, double- blind, controlled study PEP-3 KLH conjugated vaccine III Newly diagnosed
of rindopepimut/GM-CSF with adjuvant temozolomide in with GM-CSF
patients with newly diagnosed, surgically resected, EGFRvIII-
positive GBM
NCT03422094 A pilot study to assess the safety, feasibility, and Neovax with poly-ICLC, Active, Newly diagnosed
immunogenicity of a neoantigen-based personalized vaccine nivolumab, and ipilimumab recruiting
combined with immune checkpoint blockade therapy in
patients with newly diagnosed, unmethylated GBM
NCT03223103 Phase I study of tumor treatment fields and a personalized MTA-based vaccine with poly- Active, not Newly diagnosed
mutation-derived tumor vaccine in patients with newly ICLC and TTF recruiting
diagnosed GBM
NCT02455557 A Phase II study of the safety and efficacy of SVN53-67/M57- SVN53-67-KLH peptide vaccine Active, not Newly diagnosed
KLH (SurVaxM) in survivin-positive newly diagnosed GBM with GM-CSF recruiting
GBM: glioblastoma multiforme; GM-CSF: granulocyte-macrophage colony-stimulating factor; KLH: keyhole limpet hemocyanin;
EGFR: epidermal growth factor receptor; TTF: tumor treating fields; poly-ICLC: polyriboinosinic-polyribocytidylic acid-polylysine
carboxymethylcellulose
One of the most successful tumor-specific antigen peptide vaccines against GBM, rindopepimut, uses the
[17]
EGFRvIII peptide which is expressed in 25%-64% of GBM patients . Rindopepimut is commonly co-
administered with the keyhole limpet hemocyanin (KLH) and granulocyte-macrophage colony-stimulating
factor (GM-CSF) adjuvants. Adjuvants are commonly used to enhance cross-presentation of antigens to
improve immunogenicity. A promising phase II trial (ACTIVATe) conducted with rindopepimut and TMZ
treatment demonstrated a median OS of 26.0 months [Table 4]. This compared well to controls with a
median OS of only 15.0 months (TMZ treatment). Antibody formation against the EGFRvIII peptide was
observed in a small subset of patients which correlated with improved median OS, 47.7 months as compared
[73]
to 22.8 months. However, EGFRvIII negative tumor recurrence was observed in 82% of patients . A larger,
phase III clinical trial (ACT IV) was later performed that did not show significant improvement in OS
[74]
following rindopepimut treatment . KLH, an adjuvant given with the peptide vaccine, was administered
to the phase III control group but not the phase II control group. This suggests that the improved OS may
be attributable to the immunogenicity generated by KLH alone and not the peptide. Additionally, at larger
scales, peptide vaccinations may be limited by heterogeneity of the patient population. Co-administration of
rindopepimut with bevacizumab, however, improved OS for patients which suggests combination with anti-
[75]
angiogenic therapies may improve efficacy of immunotherapies .