Page 400 - Read Online
P. 400
Page 12 of 16 Pearce et al. Neuroimmunol Neuroinflammation 2018;5:47 I http://dx.doi.org/10.20517/2347-8659.2018.46
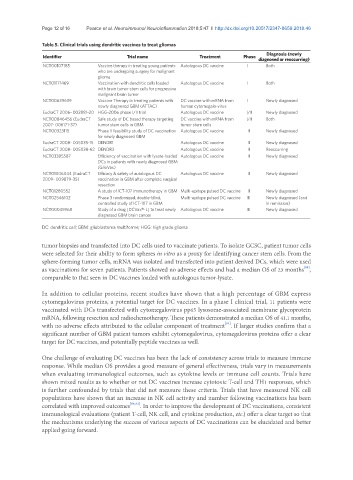
Table 5. Clinical trials using dendritic vaccines to treat gliomas
Identifier Trial name Treatment Phase Diagnosis (newly
diagnosed or reoccurring)
NCT00107185 Vaccine therapy in treating young patients Autologous DC vaccine I Both
who are undergoing surgery for malignant
glioma
NCT01171469 Vaccination with dendritic cells loaded Autologous DC vaccine I Both
with brain tumor stem cells for progressive
malignant brain tumor
NCT00639639 Vaccine Therapy in treating patients with DC vaccine with mRNA from I Newly diagnosed
newly diagnosed GBM (ATTAC) human cytomegalo-virus
EudraCT 2006- 002881-20 HGG-2006 phase I/II trial Autologous DC vaccine I/II Newly diagnosed
NCT00846456 (EudraCT Safe study of DC based therapy targeting DC vaccine with mRNA from I/II Both
2007- 006171-37) tumor stem cells in GBM tumor stem cells
NCT00323115 Phase II feasibility study of DC vaccination Autologous DC vaccine II Newly diagnosed
for newly diagnosed GBM
EudraCT 2008- 005035-15 DENDR1 Autologous DC vaccine II Newly diagnosed
EudraCT 2008- 005038-62 DENDR2 Autologous DC vaccine II Reoccurring
NCT03395587 Efficiency of vaccination with lysate-loaded Autologous DC vaccine II Newly diagnosed
DCs in patients with newly diagnosed GBM
(GlioVax)
NCT01006044 (EudraCT Efficacy & safety of autologous DC Autologous DC vaccine II Newly diagnosed
2009- 009879-35) vaccination in GBM after complete surgical
resection
NCT01280552 A study of ICT-107 immunotherapy in GBM Multi-epitope pulsed DC vaccine II Newly diagnosed
NCT02546102 Phase 3 randomized, double-blind, Multi-epitope pulsed DC vaccine III Newly diagnosed (and
controlled study of ICT-107 in GBM in remission)
NCT00045968 Study of a drug [DCVax®-L] to treat newly Autologous DC vaccine III Newly diagnosed
diagnosed GBM brain cancer
DC: dendritic cell; GBM: glioblastoma multiforme; HGG: high grade glioma
tumor biopsies and transfected into DC cells used to vaccinate patients. To isolate GCSC, patient tumor cells
were selected for their ability to form spheres in vitro as a proxy for identifying cancer stem cells. From the
sphere-forming tumor cells, mRNA was isolated and transfected into patient derived DCs, which were used
[95]
as vaccinations for seven patients. Patients showed no adverse effects and had a median OS of 23 months ,
comparable to that seen in DC vaccines loaded with autologous tumor-lysate.
In addition to cellular proteins, recent studies have shown that a high percentage of GBM express
cytomegalovirus proteins, a potential target for DC vaccines. In a phase I clinical trial, 11 patients were
vaccinated with DCs transfected with cytomegalovirus pp65 lysosome-associated membrane glycoprotein
mRNA, following resection and radiochemotherapy. These patients demonstrated a median OS of 41.1 months,
[94]
with no adverse effects attributed to the cellular component of treatment . If larger studies confirm that a
significant number of GBM patient tumors exhibit cytomegalovirus, cytomegalovirus proteins offer a clear
target for DC vaccines, and potentially peptide vaccines as well.
One challenge of evaluating DC vaccines has been the lack of consistency across trials to measure immune
response. While median OS provides a good measure of general effectiveness, trials vary in measurements
when evaluating immunological outcomes, such as cytokine levels or immune cell counts. Trials have
shown mixed results as to whether or not DC vaccines increase cytotoxic T-cell and TH1 responses, which
is further confounded by trials that did not measure these criteria. Trials that have measured NK cell
populations have shown that an increase in NK cell activity and number following vaccinations has been
correlated with improved outcomes [86,92] . In order to improve the development of DC vaccinations, consistent
immunological evaluations (patient T-cell, NK cell, and cytokine production, etc.) offer a clear target so that
the mechanisms underlying the success of various aspects of DC vaccinations can be elucidated and better
applied going forward.