Page 384 - Read Online
P. 384
Page 14 of 18 Yelton et al. Neuroimmunol Neuroinflammation 2018;5:46 I http://dx.doi.org/10.20517/2347-8659.2018.58
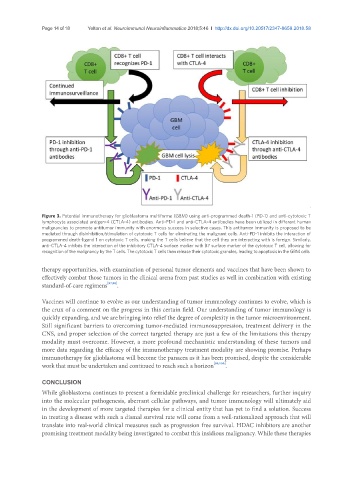
Figure 3. Potential immunotherapy for glioblastoma multiforme (GBM) using anti-programmed death-1 (PD-1) and anti-cytotoxic T
lymphocyte associated antigen-4 (CTLA-4) antibodies. Anti-PD-1 and anti-CTLA-4 antibodies have been utilized in different human
malignancies to promote antitumor immunity with enormous success in selective cases. This antitumor immunity is proposed to be
mediated through disinhibition/stimulation of cytotoxic T cells for eliminating the malignant cells. Anti-PD-1 inhibits the interaction of
programmed death-ligand 1 on cytotoxic T cells, making the T cells believe that the cell they are interacting with is foreign. Similarly,
anti-CTLA-4 inhibits the interaction of the inhibitory CTLA-4 surface marker with B7 surface marker of the cytotoxic T cell, allowing for
recognition of the malignancy by the T cells. The cytotoxic T cells then release their cytotoxic granules, leading to apoptosis in the GBM cells
therapy opportunities, with examination of personal tumor elements and vaccines that have been shown to
effectively combat those tumors in the clinical arena from past studies as well in combination with existing
standard-of-care regimens [97,98] .
Vaccines will continue to evolve as our understanding of tumor immunology continues to evolve, which is
the crux of a comment on the progress in this certain field. Our understanding of tumor immunology is
quickly expanding, and we are bringing into relief the degree of complexity in the tumor microenvironment.
Still significant barriers to overcoming tumor-mediated immunosuppression, treatment delivery in the
CNS, and proper selection of the correct targeted therapy are just a few of the limitations this therapy
modality must overcome. However, a more profound mechanistic understanding of these tumors and
more data regarding the efficacy of the immunotherapy treatment modality are showing promise. Perhaps
immunotherapy for glioblastoma will become the panacea as it has been promised, despite the considerable
work that must be undertaken and continued to reach such a horizon [99,100] .
CONCLUSION
While glioblastoma continues to present a formidable preclinical challenge for researchers, further inquiry
into the molecular pathogenesis, aberrant cellular pathways, and tumor immunology will ultimately aid
in the development of more targeted therapies for a clinical entity that has yet to find a solution. Success
in treating a disease with such a dismal survival rate will come from a well-rationalized approach that will
translate into real-world clinical measures such as progression free survival. HDAC inhibitors are another
promising treatment modality being investigated to combat this insidious malignancy. While these therapies