Page 311 - Read Online
P. 311
Toyoda. Neuroimmunol Neuroinflammation 2018;5:40 I http://dx.doi.org/10.20517/2347-8659.2018.48 Page 5 of 8
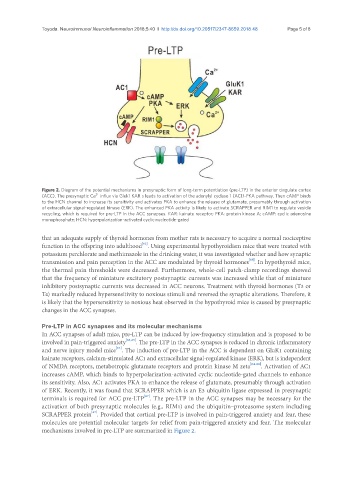
Figure 2. Diagram of the potential mechanisms in presynaptic form of long-term potentiation (pre-LTP) in the anterior cingulate cortex
2+
(ACC). The presynaptic Ca influx via Gluk1 KAR s leads to activation of the adenylyl cyclase 1 (AC1)-PKA pathway. Then cAMP binds
to the HCN channel to increase its sensitivity and activates PKA to enhance the release of glutamate, presumably through activation
of extracellular signal-regulated kinase (ERK). The enhanced PKA activity is likely to activate SCRAPPER and RIM1 to regulate vesicle
recycling, which is required for pre-LTP in the ACC synapses. KAR: kainate receptor; PKA: protein kinase A; cAMP: cyclic adenosine
monophosphate; HCN: hyperpolarization-activated cyclic nucleotide-gated
that an adequate supply of thyroid hormones from mother rats is necessary to acquire a normal nociceptive
[62]
function in the offspring into adulthood . Using experimental hypothyroidism mice that were treated with
potassium perchlorate and methimazole in the drinking water, it was investigated whether and how synaptic
[63]
transmission and pain perception in the ACC are modulated by thyroid hormones . In hypothyroid mice,
the thermal pain thresholds were decreased. Furthermore, whole-cell patch-clamp recordings showed
that the frequency of miniature excitatory postsynaptic currents was increased while that of miniature
inhibitory postsynaptic currents was decreased in ACC neurons. Treatment with thyroid hormones (T3 or
T4) markedly reduced hypersensitivity to noxious stimuli and reversed the synaptic alterations. Therefore, it
is likely that the hypersensitivity to noxious heat observed in the hypothyroid mice is caused by presynaptic
changes in the ACC synapses.
Pre-LTP in ACC synapses and its molecular mechanisms
In ACC synapses of adult mice, pre-LTP can be induced by low-frequency stimulation and is proposed to be
involved in pain-triggered anxiety [64,65] . The pre-LTP in the ACC synapses is reduced in chronic inflammatory
[64]
and nerve injury model mice . The induction of pre-LTP in the ACC is dependent on GluK1 containing
kainate receptors, calcium-stimulated AC1 and extracellular signal-regulated kinase (ERK), but is independent
of NMDA receptors, metabotropic glutamate receptors and protein kinase M zeta [64-66] . Activation of AC1
increases cAMP, which binds to hyperpolarization-activated cyclic nucleotide-gated channels to enhance
its sensitivity. Also, AC1 activates PKA to enhance the release of glutamate, presumably through activation
of ERK. Recently, it was found that SCRAPPER which is an E3 ubiquitin ligase expressed in presynaptic
[67]
terminals is required for ACC pre-LTP . The pre-LTP in the ACC synapses may be necessary for the
activation of both presynaptic molecules (e.g., RIM1) and the ubiquitin–proteasome system including
[67]
SCRAPPER protein . Provided that cortical pre-LTP is involved in pain-triggered anxiety and fear, these
molecules are potential molecular targets for relief from pain-triggered anxiety and fear. The molecular
mechanisms involved in pre-LTP are summarized in Figure 2.